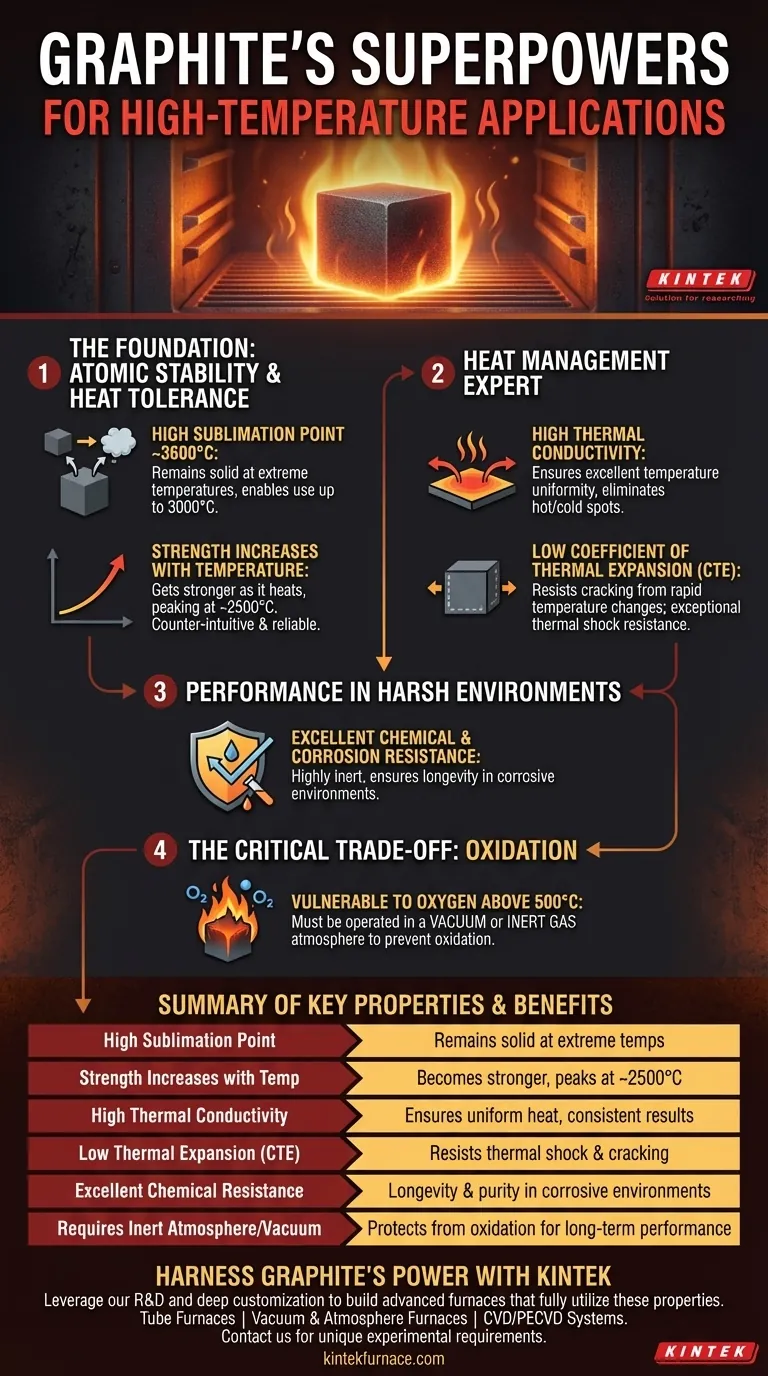
In high-temperature applications, graphite's suitability is not based on a single trait, but on a combination of unique thermal and mechanical properties. It can maintain its structural integrity at temperatures up to 3000°C, has a high thermal conductivity for uniform heating, and possesses a low coefficient of thermal expansion, which prevents it from cracking under extreme temperature changes.
The critical insight is that graphite doesn't just tolerate extreme heat; its mechanical properties actually improve with rising temperatures, all while remaining dimensionally stable. This counter-intuitive behavior makes it uniquely reliable for the most demanding thermal environments.

The Foundation: Atomic Structure and Thermal Stability
The core reasons for graphite's performance are rooted in its fundamental structure and how it behaves at extreme temperatures.
A High Sublimation Point
Unlike most materials that melt into a liquid, graphite sublimates, turning directly from a solid to a gas at atmospheric pressure around 3,600°C.
This exceptionally high phase-change temperature gives it a massive operational window, allowing it to remain a stable solid in applications like furnaces and crucibles that operate at temperatures up to 3,000°C.
A Unique Strength Profile
Unlike virtually all other materials, which weaken and soften as they get hotter, graphite's mechanical strength actually increases with temperature.
Its strength continues to rise until it peaks at approximately 2,500°C. This means that as an industrial furnace gets hotter, its graphite components become more robust, not less.
How Graphite Manages Extreme Heat
Beyond merely surviving high temperatures, graphite excels at managing thermal energy, which is critical for consistent and controllable processes.
High Thermal Conductivity
Graphite is an excellent thermal conductor, meaning it transfers heat very efficiently.
In applications like heating elements or crucibles, this ensures excellent temperature uniformity. It eliminates "hot spots" and "cold spots," leading to more consistent material processing and repeatable results.
Low Coefficient of Thermal Expansion (CTE)
Graphite expands and contracts very little when heated or cooled. This property is known as a low coefficient of thermal expansion (CTE).
This minimal expansion prevents the buildup of internal stresses during rapid temperature changes. As a result, graphite is exceptionally resistant to thermal shock and is far less likely to crack or degrade from constant heat cycling.
Performance in Harsh Environments
Industrial processes are rarely just hot; they are often chemically aggressive as well.
Excellent Chemical and Corrosion Resistance
Graphite is a highly inert material that demonstrates high resistance to corrosion and chemical attack, particularly from acids, alkalis, and solvents.
This chemical stability ensures longevity and purity in applications where the graphite components are exposed to reactive materials, such as in metal smelting or chemical synthesis.
Understanding the Primary Trade-off: Oxidation
While graphite's properties are exceptional, it has one significant limitation that is critical to manage in high-temperature applications.
Vulnerability to Oxygen
Graphite is simply a form of carbon, and at elevated temperatures, it will react with oxygen in the air and oxidize, effectively burning away.
This reaction begins to occur at a meaningful rate around 500°C.
The Need for a Controlled Atmosphere
To use graphite successfully at high temperatures, it must be operated in a vacuum or an inert (non-reactive) gas atmosphere, such as argon or nitrogen.
Protecting the graphite from oxygen is the single most important factor in ensuring its longevity and performance in furnaces and other thermal systems.
Making the Right Choice for Your Application
To leverage graphite effectively, align its key properties with the primary goal of your process.
- If your primary focus is maximum temperature and structural integrity: Rely on graphite's high sublimation point and its unique ability to get stronger as it gets hotter.
- If your primary focus is thermal uniformity and rapid cycling: Leverage its high thermal conductivity to distribute heat evenly and its low CTE to prevent cracking from thermal shock.
- If your primary focus is longevity in a non-oxidizing environment: Capitalize on its exceptional chemical resistance and overall stability to ensure a long operational life.
Ultimately, when properly managed, graphite provides a level of predictable, robust performance in extreme heat that few other materials can match.
Summary Table:
| Key Property | Benefit for High-Temperature Applications |
|---|---|
| High Sublimation Point (~3600°C) | Remains solid at extreme temperatures, enabling use up to 3000°C. |
| Strength Increases with Temperature | Becomes mechanically stronger as the environment gets hotter, peaking around 2500°C. |
| High Thermal Conductivity | Ensures excellent temperature uniformity, eliminating hot spots for consistent results. |
| Low Thermal Expansion (CTE) | Resists cracking from rapid temperature changes, providing exceptional thermal shock resistance. |
| Excellent Chemical Resistance | Highly inert, offering longevity and purity in corrosive environments. |
| Requires Inert Atmosphere/Vacuum | Must be protected from oxidation above 500°C for long-term performance. |
Ready to Harness the Power of Graphite in Your High-Temperature Processes?
Graphite's unparalleled thermal stability is the foundation of reliable high-temperature equipment. At KINTEK, we leverage our exceptional R&D and in-house manufacturing to build advanced furnaces that fully utilize these properties. Our product line—including Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—is engineered for superior performance and durability.
Our strong deep customization capability ensures we can precisely meet your unique experimental requirements. Whether your priority is maximum temperature, thermal uniformity, or longevity in a controlled atmosphere, we have the solution.
Contact us today using the form below to discuss how our high-temperature furnace solutions can enhance your lab's capabilities and drive your research forward.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- Why is a high-performance muffle furnace required for the calcination of nanopowders? Achieve Pure Nanocrystals
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control



















