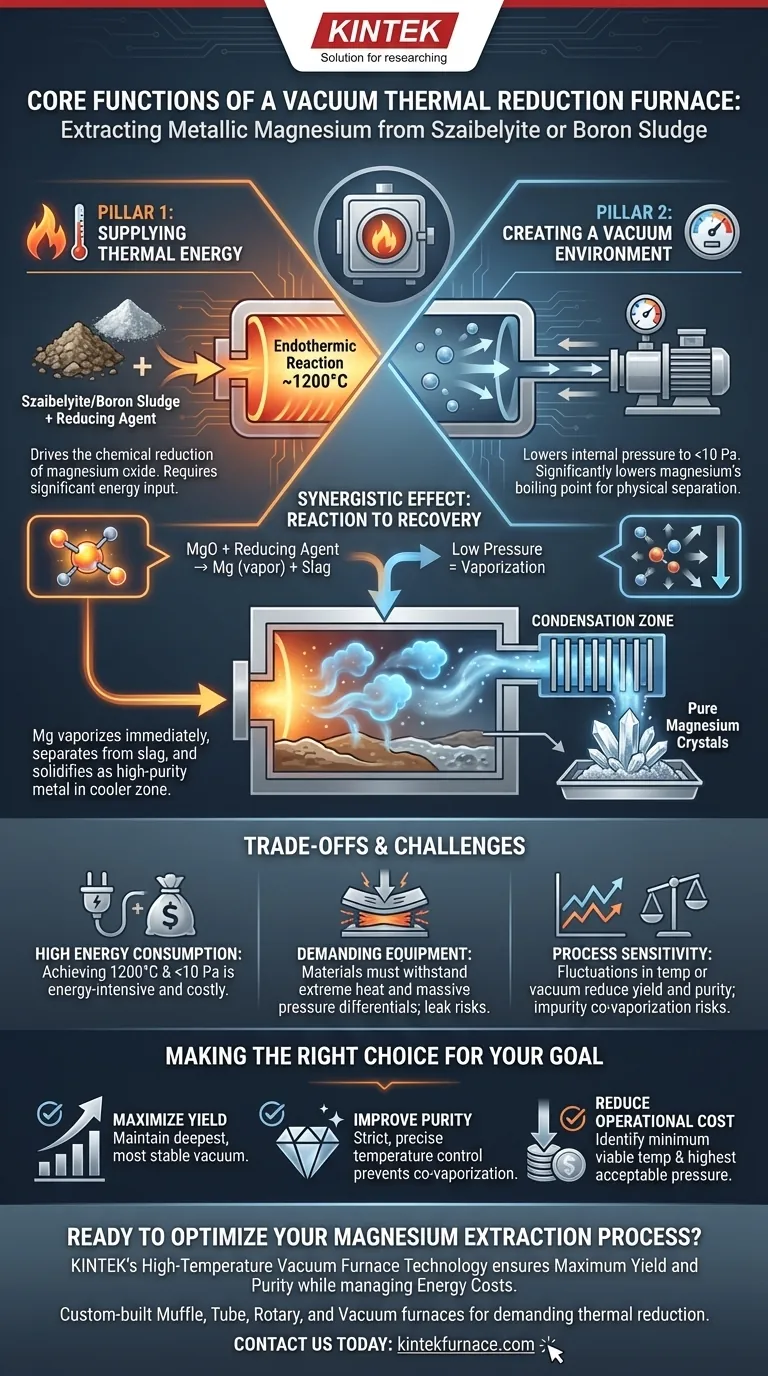
In essence, a vacuum thermal reduction furnace performs two critical and simultaneous functions to extract metallic magnesium from materials like szaibelyite or boron sludge. It provides the high temperature necessary to drive the chemical reduction of magnesium oxide, and it creates a high-vacuum environment to physically separate the newly formed magnesium product from the remaining slag.
The furnace's core purpose is not just to facilitate a chemical reaction, but to fundamentally alter the physical properties of the product—specifically, magnesium's boiling point—to make an otherwise difficult separation process efficient and effective.

The Dual Pillars of Magnesium Extraction
The entire process hinges on the furnace's ability to precisely manage two environmental conditions: heat and pressure. These two functions work in concert to both create the magnesium and then immediately purify it.
Function 1: Supplying Thermal Energy for Reduction
The conversion of magnesium oxide (the form of magnesium in the source material) into metallic magnesium is an endothermic reaction. This means it requires a significant and continuous input of energy to proceed.
The furnace provides this energy by heating the raw material and a reducing agent (such as silicon or aluminum) to temperatures around 1200°C. This intense heat provides the necessary activation energy for the chemical reaction to occur at a viable rate.
Function 2: Creating a Vacuum for Physical Separation
This is the most critical function for achieving separation. At normal atmospheric pressure, metallic magnesium has a very high boiling point. However, the furnace's vacuum pumps reduce the internal pressure to below 10 Pa, a near-perfect vacuum.
This dramatic drop in pressure significantly lowers the boiling point of magnesium. Under these conditions, as soon as metallic magnesium is formed by the chemical reaction, it immediately vaporizes at the operating temperature.
The Synergistic Effect: From Reaction to Recovery
The combination of these two functions creates a highly efficient production cycle. The heat drives the reaction, and the vacuum ensures the product immediately changes state from a solid/liquid to a gas (vapor).
This magnesium vapor is physically distinct from the remaining solid and liquid slag (containing boron, silicon, and other impurities). The vapor naturally travels to a cooler area of the furnace, the condensation zone, where it cools and solidifies into a high-purity crystalline magnesium deposit, effectively separated from the waste material.
Understanding the Trade-offs and Challenges
While effective, this process is demanding and requires careful control. The furnace's functions present inherent operational challenges that must be managed.
High Energy Consumption
Achieving and maintaining both a 1200°C temperature and a vacuum below 10 Pa is extremely energy-intensive. This represents a primary operational cost and a significant engineering challenge.
Demanding Equipment Requirements
The furnace must be constructed from materials that can withstand extreme temperatures while remaining structurally sound under a massive external pressure differential. Any leaks or material failures would cause a catastrophic loss of the vacuum environment.
Process Sensitivity
The efficiency of the extraction is highly sensitive to fluctuations in both temperature and pressure. An unstable vacuum or inconsistent heating can lead to lower yields, incomplete reactions, and reduced product purity, as other elements could also begin to vaporize if the temperature is too high.
Making the Right Choice for Your Goal
Optimizing the furnace's operation depends entirely on your primary objective, whether it's maximizing output, purity, or efficiency.
- If your primary focus is maximizing yield: Maintaining the deepest and most stable vacuum possible is critical to ensure the maximum amount of magnesium vaporizes for collection.
- If your primary focus is improving purity: Strict and precise temperature control is paramount to prevent the co-vaporization of impurities that have similar vapor pressures to magnesium.
- If your primary focus is reducing operational cost: The goal is to identify the minimum viable temperature and the highest acceptable pressure (least vacuum) that still achieves the desired separation efficiency.
Ultimately, mastering the precise interplay between temperature and pressure is the key to unlocking efficient and pure magnesium recovery with this technology.
Summary Table:
| Function | Purpose | Key Condition |
|---|---|---|
| Thermal Energy | Drives the chemical reduction of magnesium oxide. | ~1200°C |
| Vacuum Environment | Lowers magnesium's boiling point for physical separation. | < 10 Pa pressure |
| Synergistic Effect | Magnesium vaporizes and is collected as pure crystals. | Vapor travels to a condensation zone |
Ready to optimize your magnesium extraction process?
At KINTEK, we understand the critical balance between temperature and vacuum pressure required for efficient, high-purity metal recovery. Our expertise in high-temperature vacuum furnace technology ensures your operation achieves maximum yield and purity while managing energy costs.
Our custom-built Muffle, Tube, Rotary, and Vacuum furnaces, including specialized CVD systems, are engineered to meet the demanding requirements of thermal reduction processes. Backed by expert R&D and manufacturing, we provide solutions tailored to your unique material and production goals.
Contact us today to discuss how a KINTEK furnace can enhance your lab or pilot plant's capabilities. Get in touch via our contact form to speak with an expert.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing



















