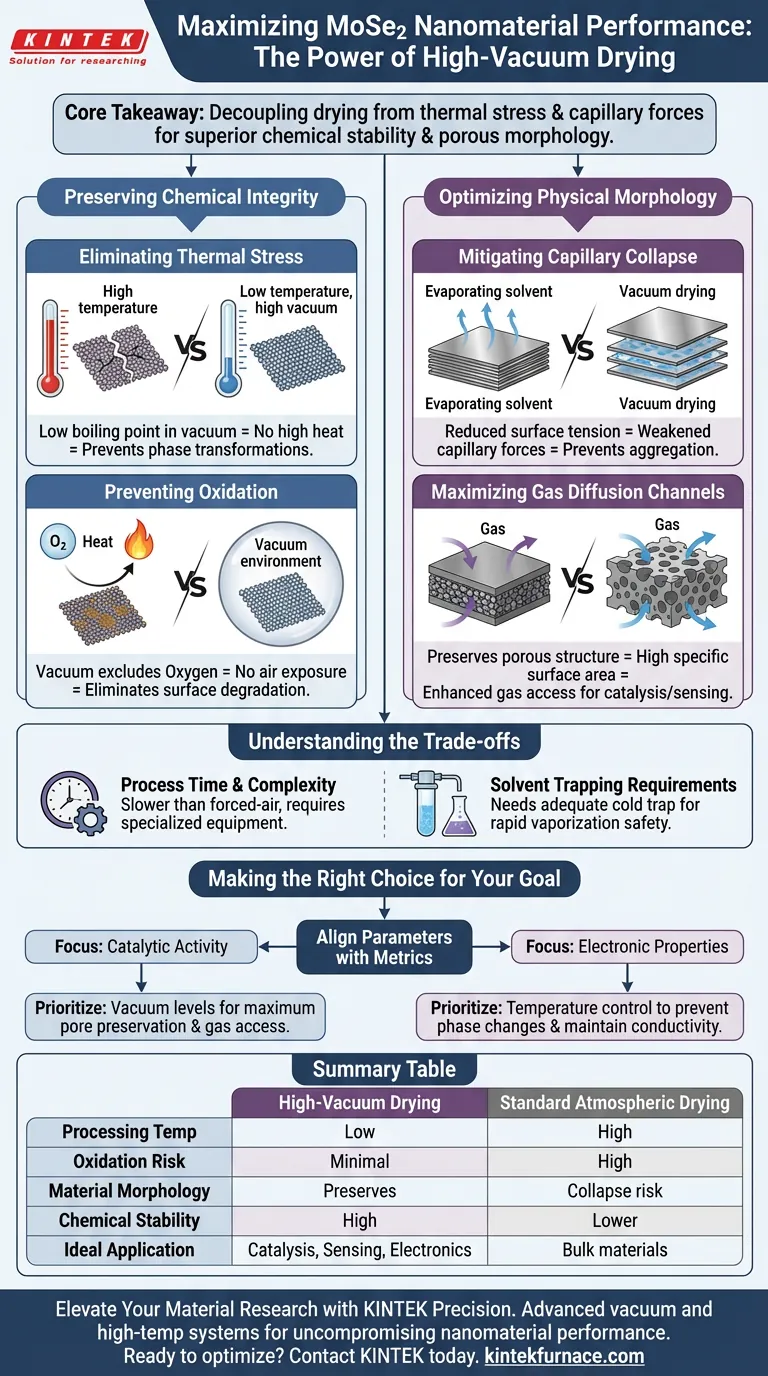
Using a high-vacuum drying oven is essential for preserving both the chemical stability and physical architecture of MoSe2 nanomaterials. This method allows for the complete removal of solvent residues at significantly reduced temperatures, preventing the oxidation and phase transformations often caused by conventional high-heat drying. Simultaneously, it protects the material's loose, porous morphology from collapsing, ensuring optimal performance in applications requiring efficient gas diffusion.
Core Takeaway By lowering the boiling point of solvents, high-vacuum drying decouples the drying process from thermal stress. This ensures the delicate MoSe2 crystal phase remains intact and oxidation-free while minimizing capillary forces to preserve the internal pore structure necessary for catalytic or electronic performance.
Preserving Chemical Integrity
Eliminating Thermal Stress
Standard drying methods rely on high temperatures to evaporate solvents, which poses a risk to heat-sensitive nanomaterials.
A high-vacuum environment significantly lowers the boiling point of solvents.
This allows you to remove residues thoroughly without exposing the MoSe2 nanosheets to temperatures that could trigger unwanted phase transformations.
preventing Oxidation
MoSe2 nanosheets can be susceptible to oxidation when exposed to heat in the presence of air.
Vacuum drying inherently excludes oxygen from the drying chamber.
By combining an oxygen-free environment with low-temperature processing, you virtually eliminate the risk of chemically degrading the material surface.
Optimizing Physical Morphology
Mitigating Capillary Collapse
During the evaporation of solvents in standard pressure conditions, surface tension creates strong capillary forces between nanosheets.
These forces can cause the material to shrink or the sheets to stack tightly together (aggregation).
Vacuum drying reduces the impact of these forces, preventing structural collapse and ensuring the material remains loose and dispersed.
Maximizing Gas Diffusion Channels
For MoSe2 to function effectively in applications like catalysis or sensing, gas molecules must be able to penetrate the material.
The high-vacuum method preserves the material's porous structure.
This directly optimizes the gas diffusion channels within the material, maintaining a high specific surface area that would otherwise be lost during high-temperature atmospheric drying.
Understanding the Trade-offs
Process Time and Complexity
While vacuum drying yields superior material quality, it is generally a slower process than forced-air convection drying.
It requires specialized equipment capable of maintaining consistent low pressure, which adds complexity to the laboratory setup compared to standard ovens.
Solvent Trapping Requirements
Because solvents boil at lower temperatures in a vacuum, they vaporize rapidly.
You must ensure your vacuum pump is equipped with an adequate cold trap to condense these vapors, preventing damage to the pump mechanism and ensuring safety in the lab.
Making the Right Choice for Your Goal
To maximize the efficacy of your MoSe2 preparation, align your drying parameters with your specific performance metrics:
- If your primary focus is Catalytic Activity: Prioritize vacuum levels that maximize pore preservation to ensure gas reactants can easily access active sites.
- If your primary focus is Electronic Properties: Prioritize temperature control within the vacuum to strictly prevent phase changes that alter conductivity.
The success of MoSe2 synthesis lies not just in the chemical reaction, but in the careful, low-stress removal of the solvent environment.
Summary Table:
| Feature | High-Vacuum Drying | Standard Atmospheric Drying |
|---|---|---|
| Processing Temperature | Low (solvent boiling point reduced) | High (requires more thermal energy) |
| Oxidation Risk | Minimal (Oxygen-free environment) | High (Heat + Oxygen exposure) |
| Material Morphology | Preserves porous, loose structure | High risk of capillary collapse/aggregation |
| Chemical Stability | High; prevents phase transformations | Lower; thermal stress may alter phases |
| Ideal Application | Catalysis, Sensing, Electronics | Bulk, heat-stable materials |
Elevate Your Material Research with KINTEK Precision
Don't let thermal stress compromise your MoSe2 synthesis. KINTEK’s advanced vacuum and high-temp systems are engineered to provide the precise atmosphere control necessary to preserve delicate nanomaterial architectures. Backed by expert R&D and manufacturing, we offer customizable Vacuum, Muffle, Tube, and CVD systems tailored for researchers who demand uncompromising material performance.
Ready to optimize your drying process? Contact KINTEK today to discuss your custom lab equipment needs.
Visual Guide
References
- Lanjuan Zhou, Dongzhi Zhang. TiO2 Nanosphere/MoSe2 Nanosheet-Based Heterojunction Gas Sensor for High-Sensitivity Sulfur Dioxide Detection. DOI: 10.3390/nano15010025
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the core function of an industrial vacuum sintering furnace in WC-8Co production? Achieve Peak Density.
- Why is a high-temperature vacuum furnace with argon protection required for sintering NiTi/HA? Ensure Phase Purity
- What are the benefits of using a vacuum furnace? Achieve Superior Purity and Precision in Heat Treatment
- What is the core role of a laboratory vacuum furnace in the carbothermic reduction process for magnesium? Creating the Ideal Environment for High-Purity Production
- How can rapid cooling (quenching) benefit the process in a vacuum furnace? Boost Efficiency and Material Properties
- What is the necessity of maintaining a vacuum level of approximately 1 Pa during the recovery of selenium-based devices?
- What additional processes can a vacuum heat treatment furnace carry out? Unlock Advanced Material Processing
- What are the primary application fields for box furnaces and vacuum furnaces? Choose the Right Furnace for Your Process



















