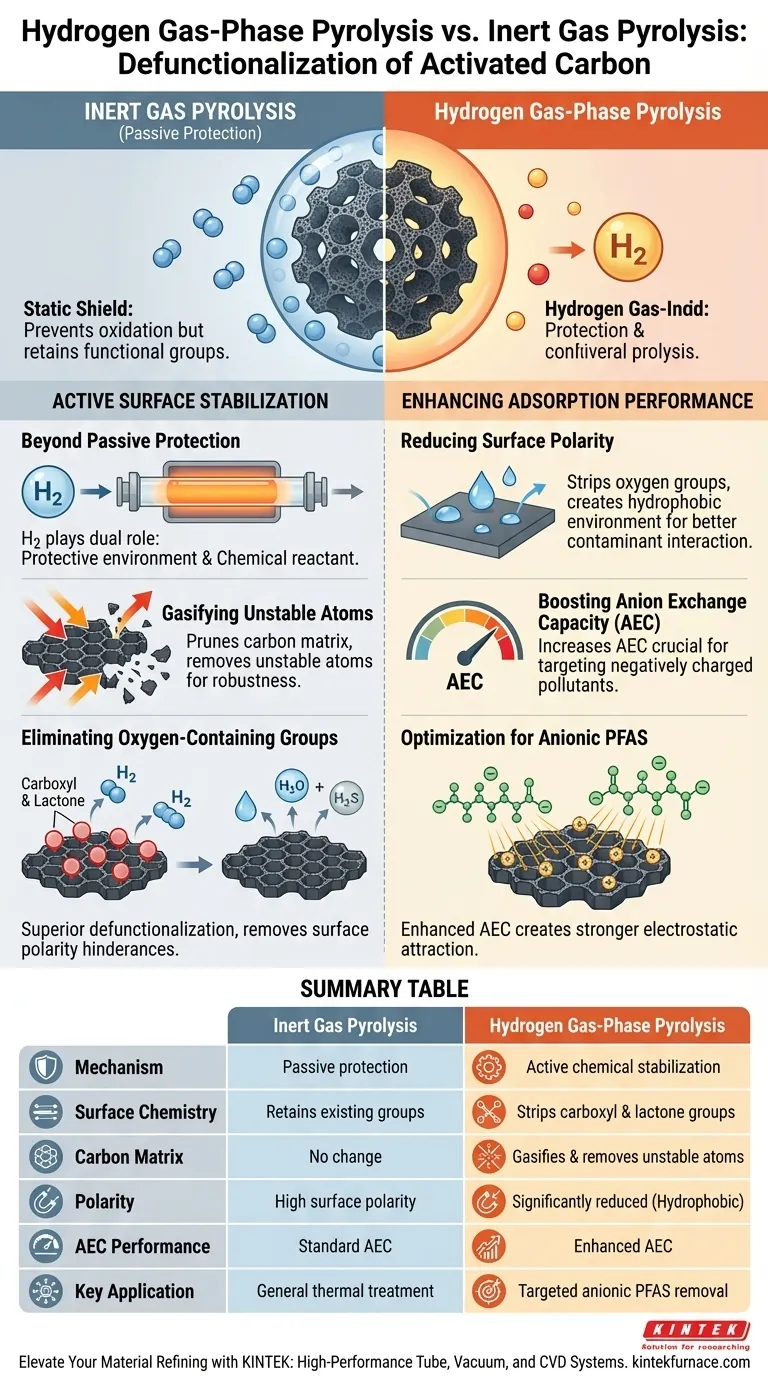
Hydrogen gas-phase pyrolysis transforms activated carbon through active chemical stabilization rather than simple passive protection. Unlike inert gas environments that merely prevent oxidation during heating, hydrogen actively engages with the material to strip away interfering functional groups and stabilize the carbon structure.
While inert gases provide a static shield, hydrogen acts as a refining agent that gasifies unstable carbon atoms and thoroughly removes oxygenated groups to maximize electrostatic performance.

Active Surface Stabilization
Beyond Passive Protection
Inert gases create a neutral environment, but hydrogen (H2) plays a dual role in a controlled atmosphere tube furnace.
It provides the necessary protective environment to prevent unwanted burning, while simultaneously acting as a chemical reactant.
Gasifying Unstable Atoms
Hydrogen stabilizes the carbon surface by targeting structural weaknesses.
It actively gasifies unstable carbon atoms, effectively pruning the carbon matrix to leave behind a more robust and chemically stable surface.
Eliminating Oxygen-Containing Groups
The primary advantage of hydrogen over inert gases is its superior ability to defunctionalize the surface.
Hydrogen drives the thorough removal of oxygen-containing functional groups, specifically carboxyl and lactone groups. These groups are responsible for surface polarity, which can hinder specific adsorption processes.
Enhancing Adsorption Performance
Reducing Surface Polarity
By stripping away carboxyl and lactone groups, hydrogen treatment significantly reduces the polarity of the activated carbon surface.
This creates a more hydrophobic environment, which alters how the carbon interacts with dissolved contaminants.
Boosting Anion Exchange Capacity
The removal of oxygenated groups directly increases the material's anion exchange capacity (AEC).
This electrochemical shift is critical for targeting specific pollutants that carry a negative charge.
Optimization for Anionic PFAS
The enhanced AEC creates a stronger electrostatic attraction toward anionic compounds.
This makes hydrogen-treated activated carbon particularly effective for the adsorption of anionic PFAS (Per- and Polyfluoroalkyl Substances), outperforming carbon treated in inert atmospheres.
Understanding the Trade-offs
Material Consumption vs. Stability
The process of "gasifying unstable carbon atoms" implies a necessary sacrifice of material to achieve stability.
Unlike inert gases, which preserve the existing carbon structure as-is, hydrogen chemically consumes the less stable portions of the carbon matrix to refine the final product.
Making the Right Choice for Your Goal
Hydrogen pyrolysis is not just a heating method; it is a chemical modification strategy.
- If your primary focus is general stability: Hydrogen offers superior structural integrity by removing unstable carbon atoms that inert gases leave behind.
- If your primary focus is PFAS removal: Hydrogen treatment is essential to maximize the electrostatic attraction required to capture anionic PFAS effectively.
By using hydrogen, you convert activated carbon from a passive adsorbent into a highly tuned material for capturing negatively charged contaminants.
Summary Table:
| Feature | Inert Gas Pyrolysis | Hydrogen Gas-Phase Pyrolysis |
|---|---|---|
| Mechanism | Passive protection (shielding) | Active chemical stabilization |
| Surface Chemistry | Retains existing functional groups | Strips carboxyl & lactone groups |
| Carbon Matrix | No change to unstable atoms | Gasifies & removes unstable atoms |
| Polarity | High surface polarity | Significantly reduced (Hydrophobic) |
| AEC Performance | Standard Anion Exchange Capacity | Enhanced AEC for better adsorption |
| Key Application | General thermal treatment | Targeted anionic PFAS removal |
Elevate Your Material Refining with KINTEK
Maximize your activated carbon’s potential with precision-engineered thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Tube, Vacuum, and CVD systems designed to handle complex hydrogen gas-phase processes safely and effectively. Whether you need standard laboratory high-temperature furnaces or fully customizable systems tailored to your unique research goals, we deliver the stability and control your work demands.
Ready to optimize your defunctionalization process? Contact KINTEK today to discuss your project requirements!
Visual Guide
References
- Md Manik Mian, Shubo Deng. Recent advances in activated carbon driven PFAS removal: structure-adsorption relationship and new adsorption mechanisms. DOI: 10.1007/s11783-025-1998-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is temperature control accuracy critical for K439B superalloy? Master 1160°C Solution Treatment
- Why is a high-precision substrate heating system required for BST thin films? Achieve Crystal Growth Success
- How does the ramp rate affect LDO properties? Master Rapid Thermal Control for 69% More Efficiency
- What is the primary purpose of 24-hour wet milling for SSBSN ceramics? Achieve Atomic-Scale Homogeneity
- Why is a blast drying oven required during zeolite modification? Ensure Structural Integrity & Precision
- What is the purpose of using a forced-air drying oven at 100 °C? Optimize Fe3O4@Fe-AC Composite Synthesis
- What is the function of a high-pressure hydrothermal reactor in hydrochar synthesis? Unlock Biomass Transformation
- How do high-temperature quenching and tempering furnaces treat AISI 304 stainless steel? Enhance Core Toughness



















