A box resistance furnace serves as the critical thermal vessel for transforming amorphous BCZT gel into crystalline oxide powders. This equipment is utilized to execute a strictly controlled two-stage heating process: a pre-firing stage at 400°C to eliminate organic materials, followed by high-temperature calcination at 800°C to synthesize the final ceramic structure.
The muffle furnace converts precursor gel into pure-phase BCZT powders by providing a stable thermal environment for chemical decomposition and reaction. It systematically removes impurities through combustion and drives the solid-state reactions necessary to form the perovskite crystal structure.
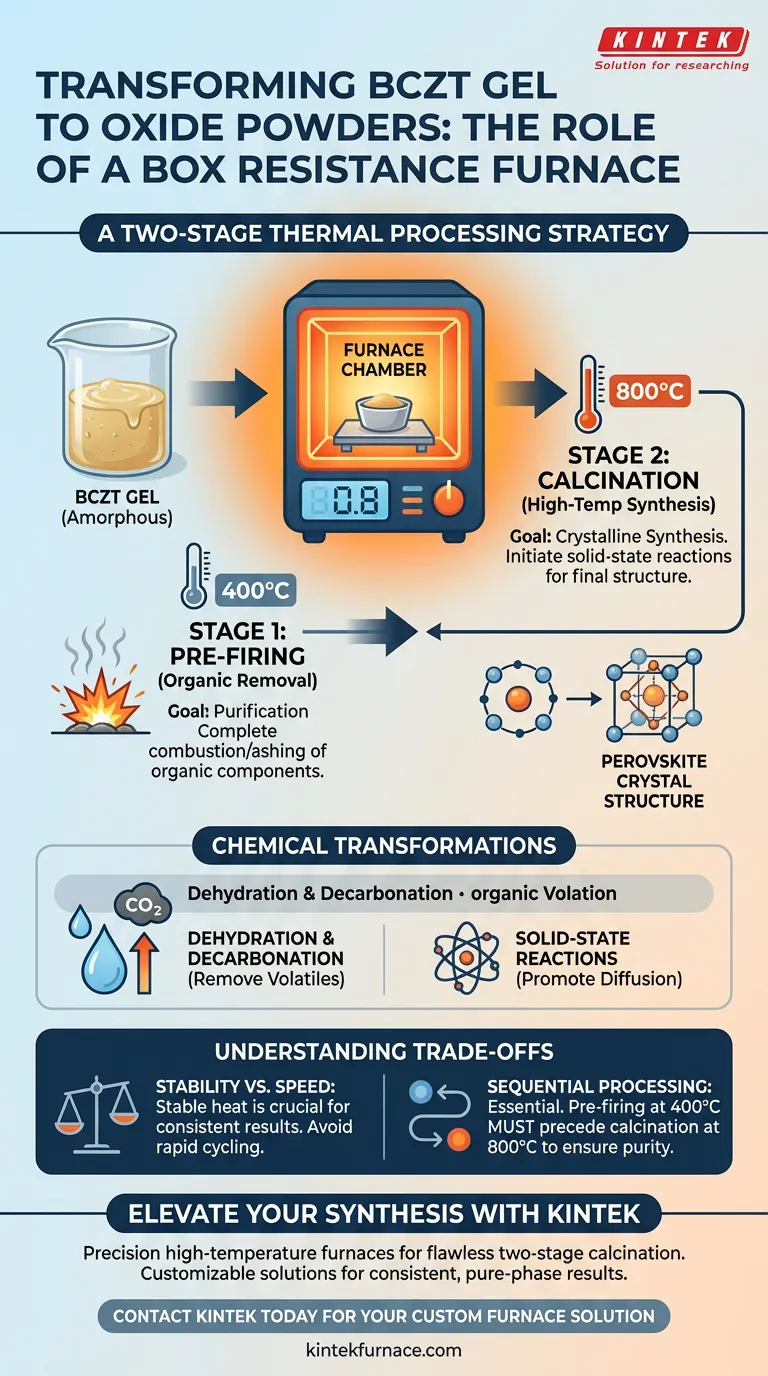
The Two-Stage Thermal Processing Strategy
To obtain high-quality oxide powders, the furnace must facilitate two distinct thermal events. The process is not a single ramp-up, but a staged approach to ensure purity and structural integrity.
Stage 1: Pre-firing for Organic Removal
The first utilization of the furnace is at a moderate temperature of 400°C.
At this stage, the primary goal is purification rather than crystallization. The furnace environment allows for the complete combustion or ashing of organic components present in the gel matrix.
Stage 2: High-Temperature Calcination
Once organics are removed, the furnace temperature is raised to 800°C for calcination.
This higher energy state is required to initiate the actual synthesis of the material. The furnace maintains a stable high-temperature environment, which is the catalyst for the final phase transformation.
Chemical Transformations Within the Chamber
The box resistance furnace does more than simply heat the material; it acts as a reactor for specific chemical changes dictated by the thermal profile.
Dehydration and Decarbonation
During the heating process, the furnace environment facilitates the removal of volatile by-products.
Specifically, the heat drives dehydration (removal of water) and decarbonation (removal of carbon-based compounds). These mechanisms are essential for preventing defects in the final powder.
Promoting Solid-State Reactions
The core function of the furnace during the 800°C stage is to promote solid-state chemical reactions.
Because the materials are not melted, the stable heat allows atoms to diffuse and rearrange within the solid state. This rearrangement is what eventually forms the pure-phase BCZT perovskite structure.
Understanding the Trade-offs
While the box resistance furnace is effective, understanding its operational constraints is vital for consistent results.
Stability vs. Speed
The reference highlights the need for a stable high-temperature environment.
Rushing the ramp rates or fluctuating the temperature can interrupt the solid-state reactions. Achieving a pure-phase perovskite structure requires patience and thermal stability, rather than rapid thermal cycling.
The Necessity of Sequential Processing
It is impossible to skip the pre-firing stage.
Attempting to jump straight to calcination temperatures would likely trap organic residues within the structure. The furnace must be utilized to fully ash organic components at 400°C before the lattice structure is formed at 800°C.
Making the Right Choice for Your Process
Successful processing of BCZT gel requires aligning your furnace protocols with the chemical requirements of the material.
- If your primary focus is material purity: Ensure the furnace is held at 400°C for sufficient time to guarantee that organic combustion and ashing are absolute before proceeding.
- If your primary focus is structural integrity: Prioritize the stability of the furnace at 800°C to maximize solid-state diffusion and ensure the formation of the full perovskite phase.
By strictly adhering to this two-step thermal profile, you ensure the transition from a raw gel to a high-performance oxide powder.
Summary Table:
| Stage | Temperature | Primary Function | Chemical Mechanism |
|---|---|---|---|
| Pre-firing | 400°C | Organic Material Removal | Combustion & Ashing |
| Calcination | 800°C | Synthesis of BCZT Powder | Solid-state Reaction |
| Environment | Stable Heat | Structural Integrity | Dehydration & Decarbonation |
Elevate Your Material Synthesis with KINTEK
Precision is paramount when transforming BCZT gels into high-performance oxide powders. KINTEK’s high-temperature box and muffle furnaces provide the thermal stability and programmed control required for flawless two-stage calcination.
Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your specific lab needs. Whether you are focusing on organic removal or complex perovskite phase formation, our systems ensure consistent, pure-phase results for every batch.
Ready to optimize your solid-state reactions? Contact KINTEK today to find your custom furnace solution.
Visual Guide
References
- Sarah Weick, M. Große. Investigating Hydrogen in Zirconium Alloys by Means of Neutron Imaging. DOI: 10.3390/ma17040781
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What role does a laboratory high-temperature muffle furnace play in converting calcified pollen into bioceramics?
- How does the temperature control system work in a muffle furnace? Ensure Precise Heating for Your Lab
- What makes muffle furnaces versatile across different industries? Discover Their Key Benefits
- What is the role of a high-temperature calcination furnace in preparing ultra-fine oxide nanopowders? Master Purity
- What role does a muffle furnace play in pharmaceuticals? Ensuring Purity and Precision in Drug Development
- How does a vacuum furnace differ from a muffle furnace in terms of operation? Choose the Right Furnace for Your Lab
- How do electrical muffle furnaces work? Unlock Precision Heating for Your Lab
- What role does a laboratory muffle furnace play in the modification of mesoporous silica carriers for drug loading?



















