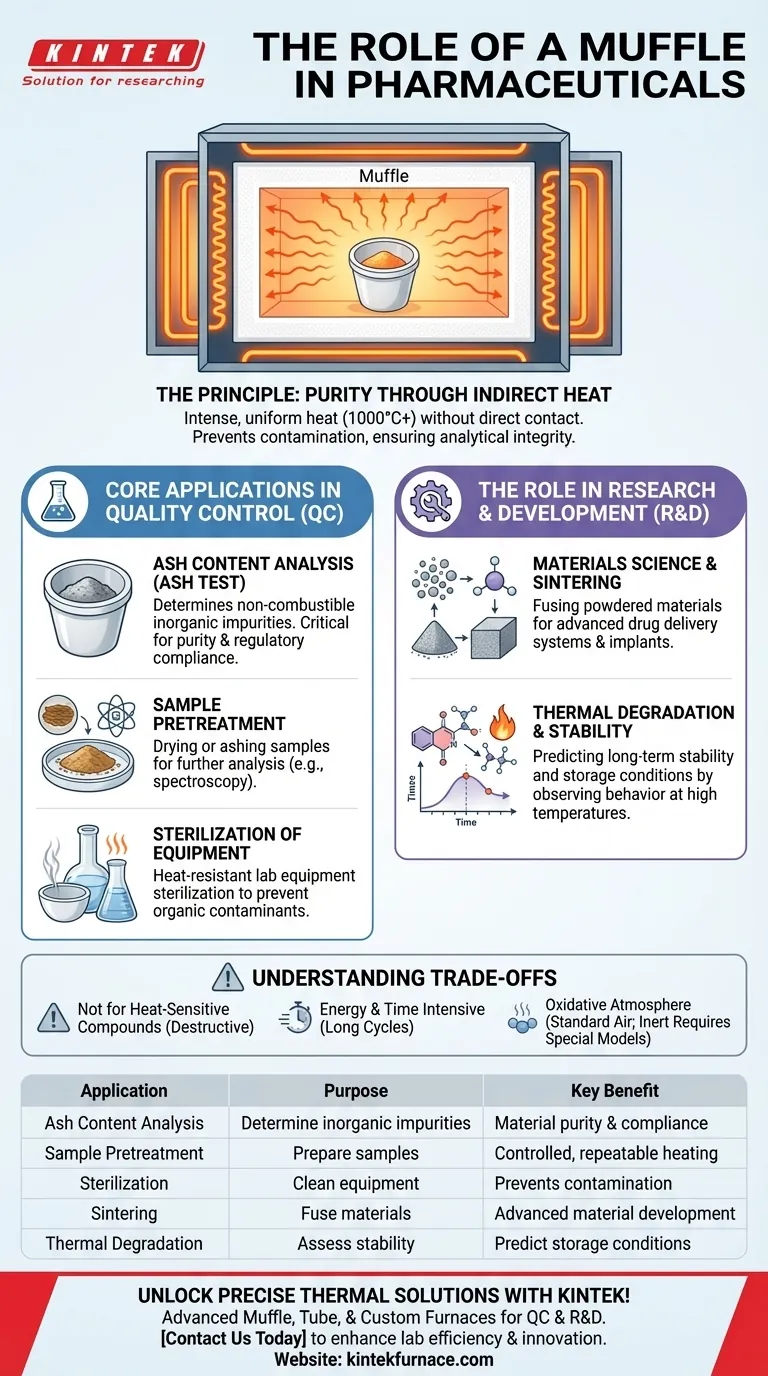
In pharmaceuticals, a muffle furnace is a specialized, high-temperature oven used for critical analytical, quality control, and research processes that demand a pure, controlled heating environment. Its primary functions include determining the non-combustible or "ash" content of a sample, preparing samples for further analysis, and developing new materials through processes like sintering and thermal degradation studies.
The core value of a muffle furnace in the pharmaceutical industry is its ability to provide intense, uniform heat without direct contact between the sample and the heating source. This "muffled" heating prevents contamination, ensuring the analytical integrity and purity required for drug development and quality assurance.

The Principle: Purity Through Indirect Heat
What Defines a Muffle Furnace?
A muffle furnace, also known as a retort furnace, is built around a central chamber (the muffle) that is insulated from the external heating elements.
The walls of this chamber radiate heat inward, bathing the sample in a uniform, high-temperature environment, often exceeding 1000°C (1832°F). The key is that the sample never touches a flame or electric coil.
Why Indirect Heat is Critical in Pharma
This design is fundamental to its role in pharmaceuticals. Direct heating methods risk introducing impurities from combustion byproducts or the heating elements themselves.
In a highly regulated field where even trace contaminants can invalidate a test or compromise a product, this isolation ensures that any changes to the sample are a result of heat alone.
Core Applications in Quality Control (QC)
Determining Non-Combustible Content (Ash Test)
One of the most common applications is ash content analysis. A sample of a drug substance or excipient is placed in the furnace and heated to a high temperature until all organic material burns away.
The material remaining is the inorganic, non-combustible "ash." This is a critical QC test to quantify inorganic impurities and confirm the identity and purity of materials according to pharmacopeial standards.
Sample Pretreatment for Analysis
Many modern analytical techniques require samples to be in a specific state. A muffle furnace is used to prepare them in a controlled, repeatable manner.
This includes completely drying samples to remove all moisture or ashing them to isolate inorganic elements for analysis by methods like atomic absorption spectroscopy.
Sterilization of Equipment
The high, dry heat of a muffle furnace is an effective method for sterilizing certain heat-resistant laboratory equipment, such as glass or metal crucibles, ensuring no organic contaminants are present before a test begins.
The Role in Research and Development (R&D)
Materials Science and Sintering
In pharmaceutical R&D, muffle furnaces are indispensable for materials science. They are used for sintering, a process where powdered materials are heated to fuse into a solid mass without melting.
This technique is vital for creating porous ceramic components used in advanced drug delivery systems or for developing new biocompatible materials for medical implants.
Thermal Degradation and Stability Studies
Researchers use muffle furnaces to subject drugs and formulations to extreme thermal stress.
By observing how a compound behaves at high temperatures, scientists can understand its degradation pathways, predict its long-term stability, and establish appropriate storage conditions.
Understanding the Trade-offs
Not for Heat-Sensitive Compounds
The primary function of a muffle furnace is high-temperature analysis, which is inherently destructive to most complex organic molecules. It is a specific tool for specific tests, not a general-purpose oven for gentle heating.
Energy and Time Consumption
These furnaces consume significant amounts of energy to reach and maintain high temperatures. Their heating and cooling cycles can be lengthy, which must be factored into laboratory workflows and throughput.
Atmosphere Considerations
A standard muffle furnace operates with an air atmosphere, which is oxidative. While this is necessary for ash testing, it can be undesirable for other processes. For applications requiring an inert environment (e.g., nitrogen or argon), more specialized and costly furnace models are necessary.
Making the Right Choice for Your Goal
Selecting and using a muffle furnace properly depends entirely on your objective.
- If your primary focus is regulatory compliance and QC: The furnace is a non-negotiable tool for pharmacopeial tests like ash content analysis, ensuring material purity.
- If your primary focus is R&D and materials innovation: Its value lies in enabling high-temperature synthesis, sintering, and thermal stress testing to develop novel materials and drug delivery systems.
- If your primary focus is general sample preparation: View it as a powerful instrument for controlled sample destruction or modification prior to elemental analysis.
Ultimately, the muffle furnace serves as a gatekeeper of purity and a catalyst for innovation, providing the precise thermal control that underpins pharmaceutical safety and advancement.
Summary Table:
| Application | Purpose | Key Benefit |
|---|---|---|
| Ash Content Analysis | Determine non-combustible impurities | Ensures material purity and regulatory compliance |
| Sample Pretreatment | Prepare samples for analysis | Provides controlled, repeatable heating |
| Sterilization | Clean heat-resistant equipment | Prevents contamination in tests |
| Sintering | Fuse powdered materials for drug delivery systems | Enables development of advanced materials |
| Thermal Degradation Studies | Assess drug stability under heat | Predicts long-term storage conditions |
Unlock precise thermal solutions for your pharmaceutical lab with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we offer advanced high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure your unique experimental needs in quality control and research are met with precision. Contact us today to enhance your lab's efficiency and innovation!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- Why is a high-performance muffle furnace required for the calcination of nanopowders? Achieve Pure Nanocrystals
- What metals cannot be heated by induction? Understanding Material Suitability for Efficient Heating
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control



















