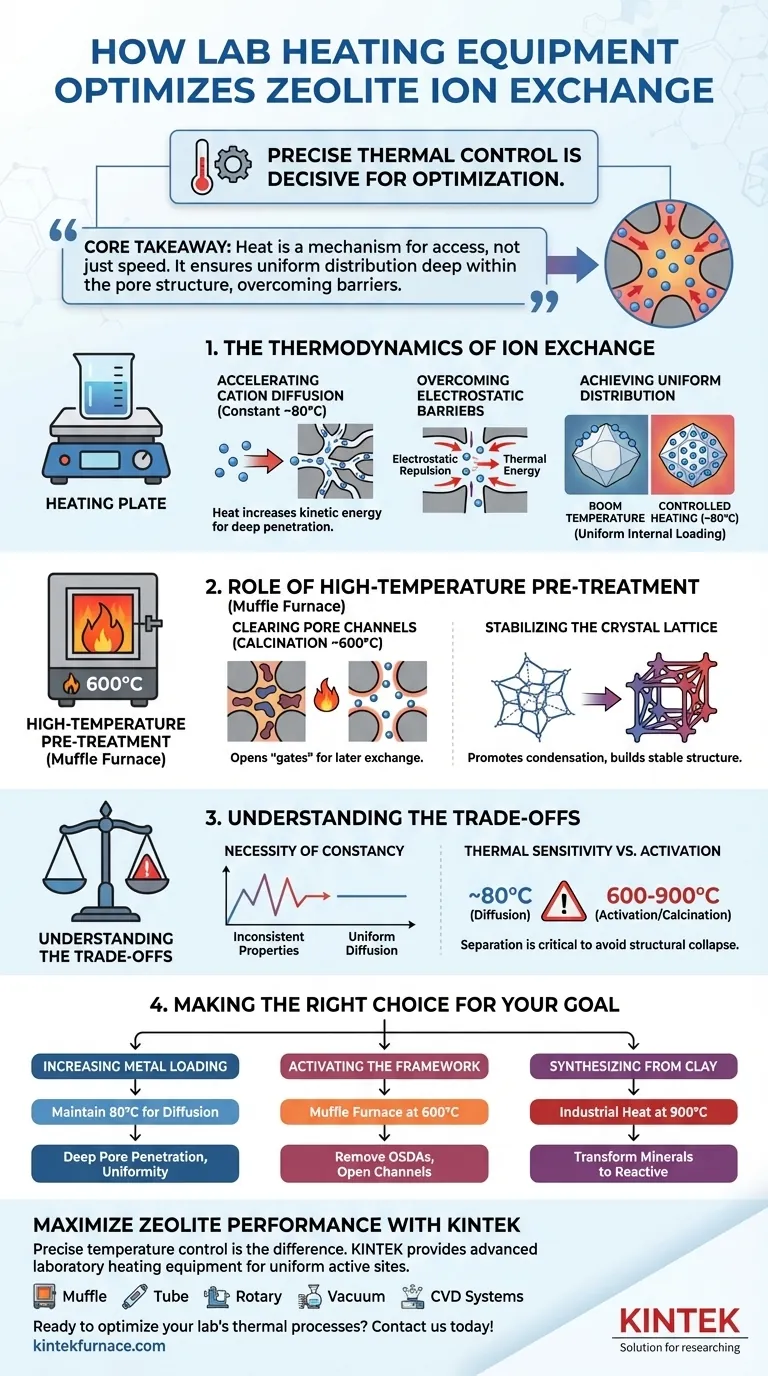
Precise thermal control using laboratory heating equipment is the decisive factor in optimizing the ion exchange process for zeolites.
By maintaining a constant temperature, typically around 80 °C, this equipment provides the necessary thermodynamic environment to accelerate the diffusion of metal cations (such as cobalt or copper) into the zeolite's structure. This thermal energy allows ions to penetrate deep into micropores they would otherwise fail to reach at room temperature.
Core Takeaway
Heat is not merely a catalyst for speed; it is a mechanism for access. By overcoming electrostatic repulsion and increasing kinetic energy, controlled heating ensures metal ions are distributed uniformly throughout the internal pore structure, rather than simply clustering on the surface.

The Thermodynamics of Ion Exchange
To understand why heating equipment is essential, you must look beyond simple chemistry and consider the physical barriers within the zeolite framework.
Accelerating Cation Diffusion
Zeolites possess complex, deep micropore structures. Without sufficient thermal energy, metal cations often struggle to navigate these narrow channels.
Heating the exchange solution increases the kinetic energy of these ions, significantly accelerating their diffusion rate. This ensures the ions can travel the full depth of the pore channels.
Overcoming Electrostatic Barriers
Ions attempting to enter the zeolite framework often face resistance. This is known as electrostatic repulsion.
The thermodynamic environment provided by a constant 80 °C heat source helps the ions overcome this repulsion. This allows for a successful exchange even in chemically resistant areas of the framework.
Achieving Uniform Distribution
Room-temperature processes often result in "surface loading," where ions crowd the outer edges of the crystal but leave the center empty.
Controlled heating ensures a uniform distribution of active sites throughout the entire crystal volume. This results in significantly higher overall metal loading and a more effective final catalyst.
The Role of High-Temperature Pre-treatment
While the ion exchange itself often occurs at moderate temperatures (80 °C), the muffle furnace plays a critical role in the steps immediately preceding exchange to make the process possible.
Clearing the Pore Channels (Calcination)
Before ion exchange can occur, the zeolite's pores must be accessible. Newly synthesized zeolites often contain organic structure-directing agents (OSDAs) blocking these paths.
A muffle furnace provides high-temperature calcination (typically 600 °C) to decompose and remove these organics. This effectively "opens the gates," releasing extra-large pore channels (such as 28-ring channels) to accept ions later.
Stabilizing the Crystal Lattice
The muffle furnace does more than just clean; it stabilizes.
Through precise programmed temperature control, the furnace creates an oxidative environment that promotes the condensation of residual hydroxyl groups. This results in a stable, four-connected crystal lattice that can withstand the rigors of the subsequent ion exchange process.
Understanding the Trade-offs
While heat is beneficial, it requires rigorous management to avoid negative outcomes.
The Necessity of Constancy
The primary reference highlights the need for a constant temperature. Fluctuations in heat can lead to uneven diffusion rates, resulting in a batch of zeolites with inconsistent catalytic properties.
Thermal Sensitivity vs. Activation
There is a distinct difference between the 80 °C used for exchange and the 600–900 °C used for calcination or clay activation.
Applying calcination-level heat (900 °C) to a solution-based ion exchange process would evaporate the solution and potentially collapse the zeolite structure. You must strictly separate thermal activation (pre-treatment) from thermal diffusion (exchange).
Making the Right Choice for Your Goal
To maximize the effectiveness of your zeolite synthesis, apply the appropriate thermal strategy to the specific stage of development.
- If your primary focus is increasing Metal Loading: maintain the ion exchange solution at a constant 80 °C to drive cations into deep micropores and overcome electrostatic repulsion.
- If your primary focus is activating the Framework: utilize a muffle furnace at 600 °C to remove organic blockages (OSDAs) and open the pore channels prior to exchange.
- If your primary focus is Synthesizing from Clay: employ industrial heat treatment at 900 °C to transform stable mineral phases into reactive components.
Controlled thermal energy transforms the zeolite from a passive filter into a highly active, uniformly loaded catalyst.
Summary Table:
| Process Stage | Typical Temperature | Key Objective | Role of Heating Equipment |
|---|---|---|---|
| Pre-treatment | 600°C - 900°C | Calcination & Activation | Removes organic templates (OSDAs) and opens pore channels. |
| Ion Exchange | ~80°C | Cation Diffusion | Overcomes electrostatic repulsion for uniform internal loading. |
| Stabilization | High-temp | Lattice Condensation | Creates a stable crystal framework to withstand chemical processes. |
| Post-Treatment | Variable | Drying & Final Calcination | Stabilizes active sites and prepares the final catalyst for use. |
Maximize Your Zeolite Performance with KINTEK
Precise temperature control is the difference between surface loading and deep-pore ion exchange. At KINTEK, we provide the advanced laboratory heating equipment necessary to achieve uniform active sites and stable crystal frameworks.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique zeolite synthesis and material science needs. Ensure your research yields consistent, high-quality results with our industry-leading thermal solutions.
Ready to optimize your lab's thermal processes? Contact us today to discuss your custom furnace requirements!
Visual Guide
References
- Konstantin Khivantsev, János Szanyi. Increasing Al-Pair Abundance in SSZ-13 Zeolite via Zeolite Synthesis in the Presence of Alkaline Earth Metal Hydroxide Produces Hydrothermally Stable Co-, Cu- and Pd-SSZ-13 Materials. DOI: 10.3390/catal14010056
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How does a muffle furnace facilitate the calcination stage of CuO/Al2O3 catalyst preparation? Optimize Active Sites
- What are the specific functions of a muffle furnace in PLxZSH ceramic treatment? Optimize Debinding & Sintering
- How does an electric laboratory furnace contribute to the glass melting process? Precision Thermal Solutions
- What applications do muffle furnaces have in ceramics? Unlock Precision Firing for Superior Results
- What are the installation and maintenance benefits of electric furnaces? Achieve Simpler, Lower-Cost Heating
- What is the use of digital muffle furnace? Unlock Precise High-Temperature Processing
- What are the safety precautions when using a box type electric furnace? Ensure Operator and Equipment Protection
- What is the core function of a muffle furnace in the preparation of g-C3N4 nanosheets? Master Material Calcination



















