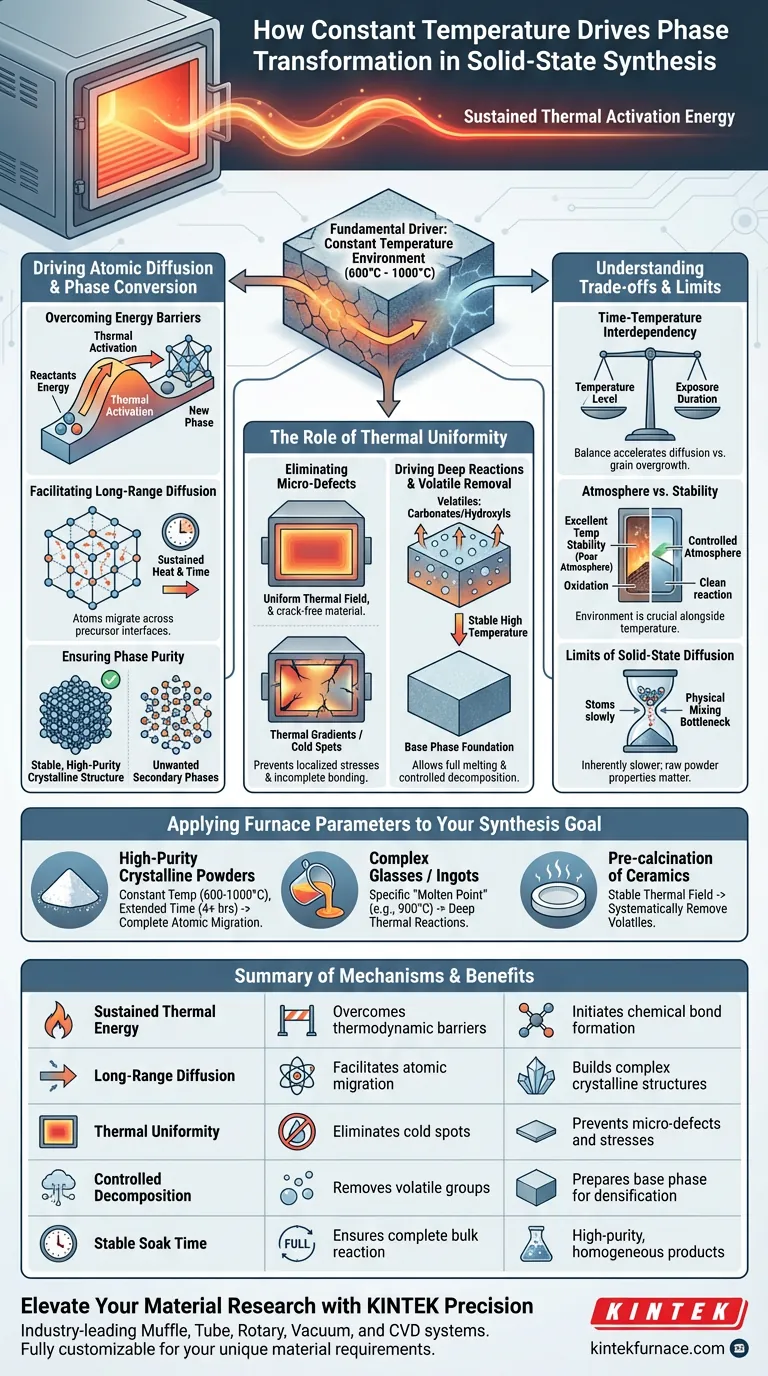
The constant temperature environment of a box high-temperature furnace is the fundamental driver of phase transformation because it provides the sustained thermal activation energy required for long-range atomic diffusion. By maintaining a stable thermal field, typically between 600°C and 1000°C, the furnace enables reactants to overcome thermodynamic barriers, allowing intermediate phases to reorganize into high-purity crystalline structures.
The core value of a constant temperature environment lies in its ability to facilitate deep thermal reactions and uniform solid-state diffusion, ensuring that chemical precursors fully convert into stable, homogenous target phases without the interference of micro-defects.

Driving Atomic Diffusion and Phase Conversion
Overcoming Thermodynamic Energy Barriers
Solid-state reactions require significant energy to break existing chemical bonds and initiate the formation of new ones. A constant temperature provides a steady stream of thermal activation energy, which is essential for reactants to surpass the "energy hump" required for phase transformation.
Facilitating Long-Range Atomic Diffusion
Unlike liquid-phase chemistry, solid-state synthesis relies on atoms moving across precursor interfaces in a solid matrix. Sustained heat over several hours ensures that these atoms have sufficient kinetic energy to migrate over long distances, which is the primary mechanism for building complex quaternary products.
Ensuring Phase Purity and Completion
Fluctuations in temperature can lead to the formation of unwanted secondary phases or incomplete reactions. A stable thermal field ensures that the entire bulk of the material reaches the necessary energy threshold simultaneously, resulting in a high-purity final product with a complete crystal structure.
The Role of Thermal Uniformity in Structural Integrity
Eliminating Micro-Defects
Rapid temperature changes or "cold spots" within a furnace can cause localized stresses or incomplete bonding. High-performance box furnaces use advanced insulation to maintain a uniform thermal field, preventing the micro-defects that typically arise from thermal gradients during the sintering process.
Driving Deep Thermal Reactions
In systems like chalcogenide glass or garnet structures (e.g., LLZTO), a constant temperature environment creates a "melting kinetic environment." This allows raw components to fully melt or react at a molecular level, ensuring that stable chemical bonds form consistently throughout the material.
Managing Volatile Component Removal
During the pre-calcination stage, a steady high temperature is used to drive off volatile groups such as carbonates or hydroxyls. This controlled decomposition is a prerequisite for forming the base phase of the material, which serves as the foundation for later densification.
Understanding the Trade-offs
Time-Temperature Interdependency
Achieving a specific phase transformation is a balance between the temperature level and the duration of exposure. While higher temperatures can accelerate diffusion, they also increase the risk of grain overgrowth or the loss of volatile elements, requiring precise programming rather than just "maximum heat."
Atmosphere vs. Temperature Stability
While temperature is the primary driver of phase change, the chemical environment (vacuum, inert, or reducing gases) also plays a role. A furnace that provides excellent temperature stability but poor atmosphere control may still fail to produce the desired phase if oxidation or contamination occurs during the long soak time.
The Limits of Solid-State Diffusion
Even with a perfectly constant temperature, solid-state diffusion is inherently slower than liquid or gas-phase reactions. This means that while the furnace provides the environment, the physical mixing and particle size of the raw powders remain critical bottlenecks that the furnace alone cannot solve.
Applying Furnace Parameters to Your Synthesis Goal
How to Apply This to Your Project
To optimize your solid-state synthesis, align your furnace settings with the specific kinetic requirements of your material system.
- If your primary focus is high-purity crystalline powders: Maintain a constant temperature within the 600°C to 1000°C range for extended periods (4+ hours) to ensure complete atomic migration across interfaces.
- If your primary focus is synthesizing complex glasses or ingots: Utilize the furnace to maintain a specific "molten point" (e.g., 900°C) to ensure deep thermal reactions and stable bond formation between ternary or quaternary elements.
- If your primary focus is the pre-calcination of ceramic electrolytes: Use the stable thermal field to systematically remove volatile components like carbonates before the final high-density sintering phase.
Success in solid-state synthesis is defined by the precision of the thermal field, as it transforms raw chemical mixtures into structured, functional materials.
Summary Table:
| Mechanism | Impact on Synthesis | Key Benefit |
|---|---|---|
| Sustained Thermal Energy | Overcomes thermodynamic barriers | Initiates chemical bond formation |
| Long-Range Diffusion | Facilitates atomic migration | Builds complex crystalline structures |
| Thermal Uniformity | Eliminates cold spots | Prevents micro-defects and stresses |
| Controlled Decomposition | Removes volatile groups (carbonates/hydroxyls) | Prepares base phase for densification |
| Stable Soak Time | Ensures complete bulk reaction | High-purity, homogeneous products |
Elevate Your Material Research with KINTEK Precision
Achieving perfect phase purity in solid-state synthesis requires uncompromising thermal stability. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the precise constant temperature environments your research demands.
Backed by expert R&D and world-class manufacturing, our lab high-temp furnaces are fully customizable to meet your unique material requirements—from chalcogenide glass synthesis to ceramic electrolyte pre-calcination.
Ready to optimize your sintering process? Contact our technical experts today to find the ideal furnace solution for your laboratory.
Visual Guide
References
- Jiadong Chen, Wenhao Sun. Navigating phase diagram complexity to guide robotic inorganic materials synthesis. DOI: 10.1038/s44160-024-00502-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What safety features are enhanced in muffle furnaces? Discover Advanced Protection for Your Lab
- How do high-precision electric furnaces facilitate microstructural transformation in aluminum alloys? Expert Insights
- How does a high-temperature muffle furnace convert shell powder to CaO? Achieve High-Purity Calcium Oxide via Calcination
- Why are muffle furnaces particularly suitable for ashing processes? Achieve Contaminant-Free Sample Analysis
- What is the alternative to a muffle furnace? Choose the Right High-Temp Furnace for Your Process
- What is the primary function of an industrial muffle furnace? Unlock High-Performance Carbon Paper Electrodes
- What are the advantages of using an Infrared Rapid Heating Furnace? Capture Transient Atomic Migrations in Steel
- What are the specific technical functions of hydrothermal autoclaves and muffle furnaces in catalyst preparation?



















