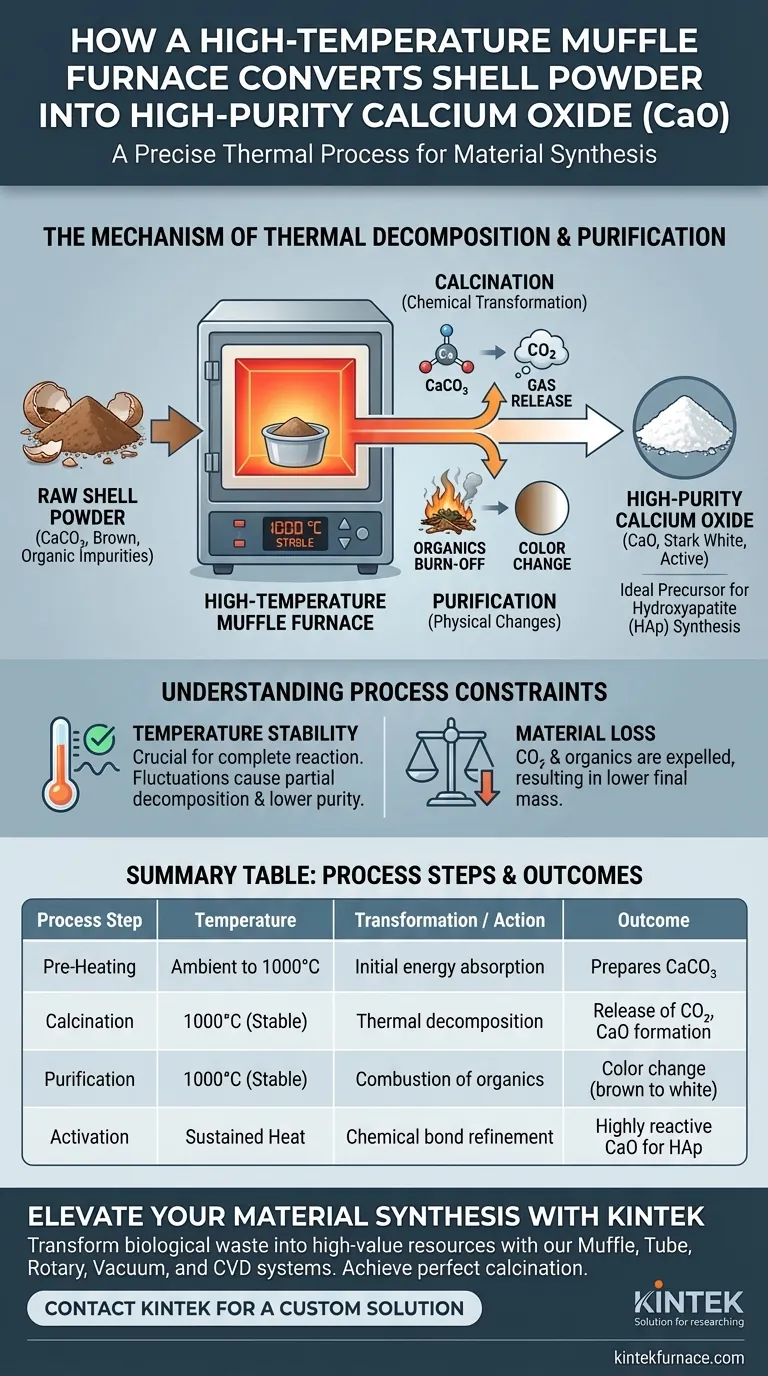
A high-temperature muffle furnace facilitates this conversion through a precise thermal process known as calcination. By maintaining a stable environment at 1000 °C, the furnace thermally decomposes the calcium carbonate ($CaCO_3$) found in raw shells. This intense heat drives off carbon dioxide and burns away organic impurities, effectively transforming the material into high-purity, active calcium oxide ($CaO$).
The muffle furnace acts as a precision purification tool, using stable high heat to strip away organic contaminants and chemically alter shell waste. The result is a clean, white, and chemically active calcium oxide powder essential for advanced material synthesis.

The Mechanism of Thermal Decomposition
Achieving Critical Temperatures
The primary function of the muffle furnace is to generate and sustain a consistent temperature of 1000 °C. This specific thermal threshold is required to provide the energy necessary to break the chemical bonds within the shell powder.
Chemical Transformation
At this temperature, the calcium carbonate ($CaCO_3$) inherent in the shells undergoes thermal decomposition. The heat forces the release of carbon dioxide ($CO_2$) gas, fundamentally altering the substance's chemical structure into calcium oxide ($CaO$).
Ensuring Complete Reaction
The stability of the muffle furnace is crucial for ensuring the reaction is uniform throughout the sample. A fluctuating temperature could lead to partial decomposition, resulting in a mixture rather than pure calcium oxide.
Purification and Physical Changes
Elimination of Organic Matter
Raw shell powder typically appears brown due to the presence of residual organic matter. The high-temperature environment of the furnace effectively combusts these organic impurities, removing them entirely from the matrix.
Visual Indicators of Purity
As the organic matter is oxidized and the chemical conversion completes, the physical appearance of the powder changes drastically. The transition from a brown powder to a stark white powder serves as a visual indicator of high purity.
Activation for Synthesis
The resulting white powder is not just pure; it is chemically "active." This reactivity makes it an ideal precursor for synthesizing complex biomaterials, specifically hydroxyapatite (HAp).
Understanding Process Constraints
Dependence on Temperature Stability
The quality of the final calcium oxide is directly tied to the furnace's ability to hold 1000 °C without deviation. If the temperature drops, the calcination may be incomplete, leaving residual calcium carbonate that compromises purity.
Material Loss Considerations
It is important to note that the process involves a reduction in mass. As carbon dioxide and organic matter are expelled into the atmosphere, the total weight of the final calcium oxide product will be lower than the initial shell powder input.
Optimizing the Calcination Process
To ensure you achieve the high-purity output required for your specific application, consider these targeted recommendations:
- If your primary focus is maximum purity: Monitor the color change carefully; ensure the furnace maintains 1000 °C until the powder shifts completely from brown to white to guarantee organic removal.
- If your primary focus is hydroxyapatite synthesis: Prioritize the stability of the thermal environment to ensure the production of fully active CaO, as incomplete calcination will inhibit the downstream synthesis of HAp.
By strictly controlling the thermal environment, you turn biological shell waste into a precise chemical resource.
Summary Table:
| Process Step | Temperature | Transformation / Action | Outcome |
|---|---|---|---|
| Pre-Heating | Ambient to 1000°C | Initial energy absorption | Prepares $CaCO_3$ for decomposition |
| Calcination | 1000°C (Stable) | Thermal decomposition of $CaCO_3$ | Release of $CO_2$ and CaO formation |
| Purification | 1000°C (Stable) | Combustion of organic matter | Color change from brown to stark white |
| Activation | Sustained Heat | Chemical bond refinement | Highly reactive CaO for HAp synthesis |
Elevate Your Material Synthesis with KINTEK
Transform biological waste into high-value chemical resources with precision thermal processing. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the strict temperature stability required for perfect calcination. Whether you are producing high-purity calcium oxide or synthesizing advanced biomaterials, our customizable lab high-temp furnaces provide the uniform heating essential for your success.
Ready to optimize your lab's thermal efficiency? Contact KINTEK today for a custom solution.
Visual Guide
References
- Charlena Charlena, Muhammad Dicky Iswara. Synthesis and Characterization of Hydroxyapatite Composites Based on Tutut (Belamya Javanica) and Magnetite by Coprecipitation as Adsorbents of Pb Metals Ion. DOI: 10.26554/sti.2025.10.1.111-122
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the primary function of a muffle furnace in birnessite preparation? Optimize High-Temp Calcination Control
- What is the application of electric muffle furnace? Achieve Precise Heat Treatment for Your Lab
- Why are muffle furnaces important in quality control? Ensure Product Integrity with Controlled Heat Testing
- What role does an air vent play in muffle furnaces? Master Atmosphere Control for Precise Results
- How is a muffle furnace utilized for AlN crystal post-processing? Optimize Surface Purity via Staged Oxidation
- What is the high temperature of a muffle furnace? Find the Right Tier for Your Application
- How are muffle furnaces classified according to control devices? Choose the Right Control for Precision Heating
- What is the function of an industrial muffle furnace in g-C3N4 synthesis? Optimize Your Thermal Polymerization



















