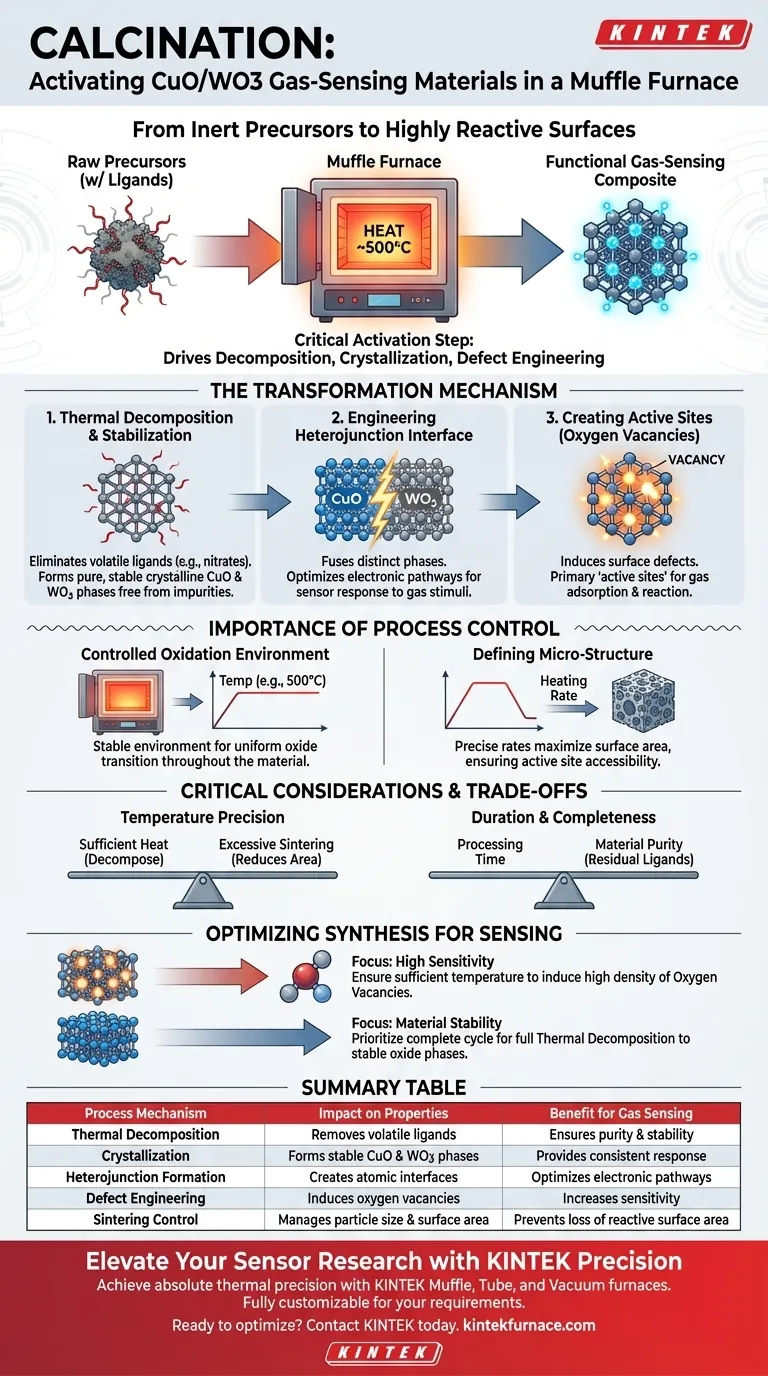
Calcination is the critical activation step that converts raw chemical precursors into a functional gas-sensing composite. In a muffle furnace, subjecting the material to high temperatures (specifically around 500°C) drives thermal decomposition, crystallizes the compounds into stable Copper Oxide (CuO) and Tungsten Oxide (WO3), and engineers the microscopic defects required for detecting gases.
The calcination process does more than simply dry the material; it fundamentally alters its atomic structure. By creating stable crystalline phases and establishing specific electronic interfaces, the furnace transforms inert precursors into a highly reactive surface capable of electron exchange.

The Transformation Mechanism
Thermal Decomposition and Stabilization
The intense heat of the muffle furnace initiates the breakdown of precursor chemicals. This process eliminates volatile organic ligands, such as nitrates or acetylacetonates, which are adsorbed on the support. What remains are pure, stable crystalline forms of CuO and WO3, free from impurities that would otherwise hinder performance.
Engineering the Heterojunction Interface
Perhaps the most vital result of calcination is the creation of heterojunctions. This occurs where the distinct phases of Copper Oxide and Tungsten Oxide meet at the atomic level. The thermal energy fuses these interfaces, optimizing the electronic pathways essential for the sensor to respond to gas stimuli.
Creating Active Sites (Oxygen Vacancies)
The thermal stress induces specific surface defects known as oxygen vacancies. Far from being flaws, these vacancies are the primary "active sites" of the material. They serve as the precise locations where target gas molecules adsorb and react, directly influencing the sensor's sensitivity.
The Importance of Process Control
Controlled Oxidation Environment
A muffle furnace provides a stable oxidation environment necessary for complete conversion. By maintaining constant temperature stages (e.g., 500°C for 2 hours), the furnace ensures the transition to oxide states is uniform throughout the material.
Defining the Micro-Structure
The heating profile dictates the final physical arrangement of the material. Precise heating rates allow the oxides to settle into a micro-structure that maximizes surface area. This "pre-forming" of the structure ensures that the active sites are accessible to gas molecules later on.
Critical Considerations and Trade-offs
Temperature Precision
The specific temperature setpoint is not arbitrary. The heat must be high enough to fully decompose the precursors and crystallize the oxides, but controlled enough to prevent excessive sintering, which would reduce surface area.
Duration and Completeness
The duration of the calcination (e.g., 2 hours) is a trade-off between processing time and material purity. Cutting this time short risks leaving behind residual ligands that block active sites, rendering the sensor ineffective.
Optimizing Material Synthesis for Sensing
To maximize the efficacy of your CuO/WO3 sensors, you must view the calcination profile as a design variable, not just a manufacturing step.
- If your primary focus is High Sensitivity: Ensure the temperature is sufficient to induce a high density of oxygen vacancies, as these are the primary sites for gas interaction.
- If your primary focus is Material Stability: Prioritize a complete calcination cycle to ensure full thermal decomposition of precursors into their most stable crystalline oxide phases.
Ultimately, the muffle furnace is the instrument used to engineer the electronic behavior of your sensor at the atomic level.
Summary Table:
| Process Mechanism | Impact on CuO/WO3 Properties | Benefit for Gas Sensing |
|---|---|---|
| Thermal Decomposition | Removes volatile ligands (nitrates/acetylacetonates) | Ensures material purity and stability |
| Crystallization | Forms stable CuO and WO3 crystalline phases | Provides consistent sensor response |
| Heterojunction Formation | Creates atomic-level interfaces between oxides | Optimizes electronic pathways for detection |
| Defect Engineering | Induces oxygen vacancies (active sites) | Increases sensitivity for gas adsorption |
| Sintering Control | Manages particle size and surface area | Prevents loss of reactive surface area |
Elevate Your Sensor Research with KINTEK Precision
Achieving the perfect balance of oxygen vacancies and crystalline stability requires absolute thermal precision. KINTEK provides high-performance Muffle, Tube, and Vacuum furnaces specifically engineered for the rigorous demands of material synthesis and calcination.
Backed by expert R&D and advanced manufacturing, our systems are fully customizable to meet your unique temperature profiles and atmospheric requirements—ensuring your CuO/WO3 composites reach their maximum sensing potential.
Ready to optimize your calcination process? Contact KINTEK today to discuss your laboratory’s high-temperature needs with our technical specialists.
Visual Guide
References
- Peishuo Wang, Xueli Yang. Engineering Hierarchical CuO/WO3 Hollow Spheres with Flower-like Morphology for Ultra-Sensitive H2S Detection at ppb Level. DOI: 10.3390/chemosensors13070250
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the main components of a muffle furnace? Key Parts for Precision High-Temp Control
- Why is precise temperature control critical when sintering 13-93 bioactive glass? Expert Thermal Management Guide
- What are the uses of muffle furnaces in calcination and sintering? Achieve Precise High-Temperature Material Transformations
- How is a muffle furnace applied in the active sulfur coating process? Achieve 155 °C Precision for Catalyst Composites
- Why are muffle furnaces popular in industrial sectors? Discover Their Key Benefits for Clean, Precise Heating
- What types of heating elements are commonly used in box furnaces? Optimize Your High-Temp Processes
- What role does a muffle furnace play in the solid-state reaction synthesis of Dy4T1-xGa12? Achieve Pure Alloy Phases
- What environmental testing applications involve muffle furnaces? Achieve Accurate Soil and Water Analysis



















