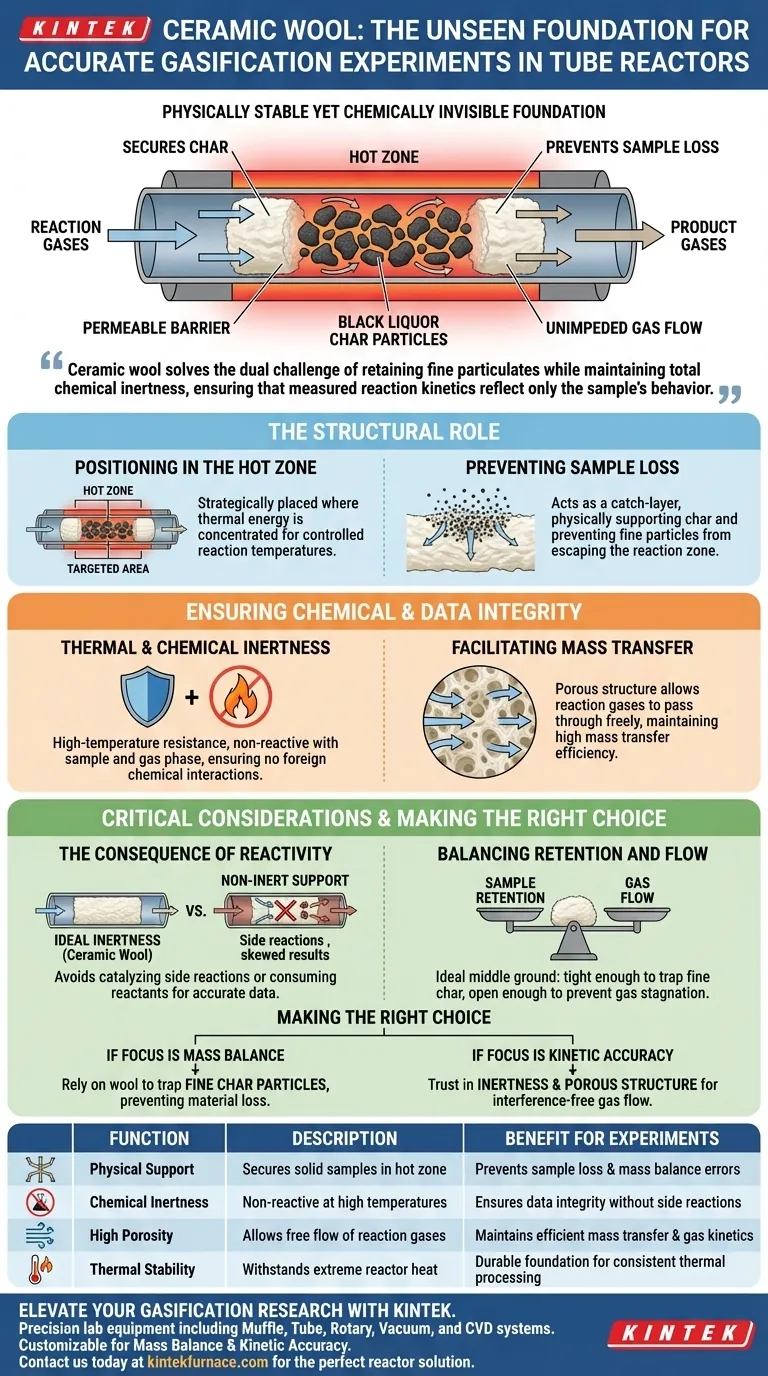
Ceramic wool acts as a physically stable yet chemically invisible foundation for solid samples within the high-temperature environment of a tube reactor. It creates a permeable barrier in the hot zone that secures black liquor char particles, preventing them from falling out of the reaction zone without impeding the flow of essential gases.
Ceramic wool solves the dual challenge of retaining fine particulates while maintaining total chemical inertness, ensuring that measured reaction kinetics reflect only the sample's behavior and not the support material.

The Structural Role of Ceramic Wool
Positioning in the Hot Zone
In gasification experiments, the reaction must occur at specific, controlled temperatures. Ceramic wool is strategically placed directly in the hot zone of the tube reactor.
This positioning ensures that the black liquor char samples are held precisely where the thermal energy is concentrated.
Preventing Sample Loss
One of the primary mechanical functions of ceramic wool is to act as a catch-layer. It physically supports the char samples and prevents fine particles from falling through the reactor tube.
Without this physical barrier, smaller particulates could escape the reaction zone, leading to incomplete data or mass balance errors.
Ensuring Chemical and Data Integrity
Thermal and Chemical Inertness
To obtain accurate gasification measurements, the support material must not become part of the experiment. Ceramic wool possesses excellent high-temperature resistance, allowing it to withstand the reactor's heat without degrading.
Furthermore, it is chemically inert. It remains non-reactive with both the solid char sample and the surrounding gas phase, ensuring no foreign chemical interactions skew the results.
Facilitating Mass Transfer
While the wool must hold the solid sample, it must not block the gas flow. Ceramic wool features a highly porous structure that allows reaction gases to pass through freely.
This porosity maintains high mass transfer efficiency, which is critical for ensuring the gasification process proceeds uninhibited.
Critical Considerations for Experimental Integrity
The Consequence of Reactivity
A common pitfall in reactor design is selecting a support material that interacts with the sample. If the support layer is not fully inert, it may catalyze side reactions or consume reactants.
Ceramic wool is specifically utilized to avoid this variable, ensuring that changes in the gas phase are attributed solely to the char gasification.
Balancing Retention and Flow
The trade-off in support material selection lies between holding the sample and allowing gas flow. A material that is too dense might hold fines well but choke the reactor.
Ceramic wool serves as the ideal middle ground, offering a matrix tight enough to trap fine char but open enough to prevent back-pressure or gas stagnation.
Making the Right Choice for Your Experiment
To ensure your gasification data is reliable, apply the specific properties of ceramic wool to your experimental goals:
- If your primary focus is Mass Balance: Rely on the ceramic wool to trap fine char particles, preventing material loss that would ruin gravimetric analysis.
- If your primary focus is Kinetic Accuracy: Trust in the chemical inertness and porous structure of the wool to allow gases to flow without interference.
By utilizing ceramic wool, you secure the physical sample without compromising the chemical validity of the reaction environment.
Summary Table:
| Function | Description | Benefit for Experiments |
|---|---|---|
| Physical Support | Secures solid samples/char in the hot zone | Prevents sample loss and mass balance errors |
| Chemical Inertness | Non-reactive at high temperatures | Ensures data integrity without side reactions |
| High Porosity | Allows free flow of reaction gases | Maintains efficient mass transfer and gas kinetics |
| Thermal Stability | Withstands extreme reactor heat | Durable foundation for consistent thermal processing |
Elevate Your Gasification Research with KINTEK
Precision in the lab starts with the right equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique experimental needs. Whether you are optimizing mass balance or ensuring kinetic accuracy, our lab high-temp furnaces provide the thermal stability your research demands.
Ready to upgrade your thermal processing? Contact us today to consult with our experts and find the perfect reactor solution for your laboratory.
Visual Guide
References
- F. Bueno, José Luis Sánchez. CO₂ Gasification of Black Liquor Char under isothermal and dynamic conditions. DOI: 10.26754/jji-i3a.202512008
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why use a graphite box in tube furnaces for Sb2Se3 annealing? Achieve Precise Crystal Growth & Vapor Control
- What is the function of an industrial-grade tube furnace? Mastering Expanded Graphite (EG) Calcination
- What are the key design features of a split tube furnace? Unlock Superior Access for Complex Experiments
- How does a high-precision tube sintering furnace contribute to the crystal structure formation of Li3-3xScxSb?
- What happens to quartz tubes in a tube furnace at temperatures above 1000°C? Understanding Devitrification and Material Limits
- How does a tube furnace ensure a controlled reaction environment? Achieve Precise Isothermal Oxidation Results
- What role does a tube furnace play in the synthesis of one-dimensional silicon nanowires (SiNWs) using CVD?
- What role does a tube furnace play in Se/NC composite synthesis? Mastering the Melt-Diffusion Method



















