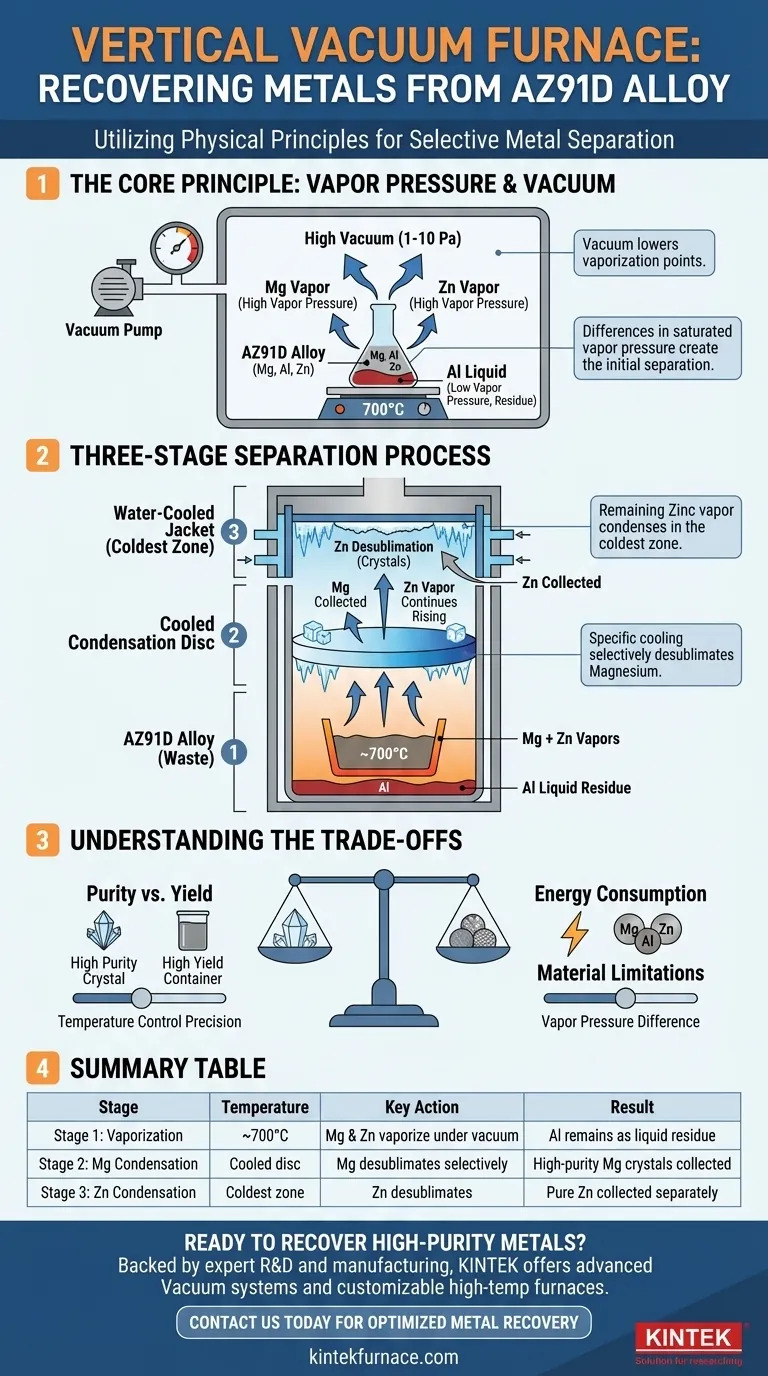
A vertical vacuum furnace separates metals by exploiting their unique boiling points under vacuum. This process leverages the principle that at a specific temperature and low pressure, some metals will turn into a gas while others remain liquid or solid. For a magnesium alloy like AZ91D heated to 700°C in a high vacuum, the high vapor pressures of magnesium and zinc cause them to vaporize, while aluminum's extremely low vapor pressure forces it to stay behind. The mixed metal vapor then rises and condenses on separate, strategically cooled surfaces, allowing for their individual collection.
The entire separation hinges on a powerful physical principle: different elements have vastly different tendencies to become a gas (vapor pressure), and a vacuum dramatically amplifies these differences. By creating a controlled temperature gradient inside the furnace, we can selectively vaporize and then re-solidify each metal in a different location.

The Core Principle: Vapor Pressure Differential
The effectiveness of vacuum distillation for separating alloys is not magic; it is a direct application of fundamental physics. Understanding the concept of vapor pressure is key to grasping how this technology works.
What is Saturated Vapor Pressure?
Every material has a natural tendency to evaporate, creating a vapor that exerts a certain pressure. This is called saturated vapor pressure.
This pressure is highly dependent on temperature. As you heat a substance, its vapor pressure increases exponentially, making it more volatile.
The Critical Role of Vacuum
The atmosphere around us exerts pressure, making it harder for liquids or solids to boil or sublimate. By pumping the air out of the furnace to create a high vacuum (1-10 Pa), we remove this opposing pressure.
This drastically lowers the temperature at which metals will vaporize. A vacuum makes it possible to "boil" metals like magnesium at a much lower, more energy-efficient temperature.
Exploiting the Differences in AZ91D
The AZ91D alloy is primarily composed of magnesium (Mg), aluminum (Al), and zinc (Zn). At the operating temperature of 700°C under vacuum, their vapor pressures are dramatically different:
- Magnesium and Zinc: Have very high vapor pressures, causing them to readily transform into a gaseous state.
- Aluminum: Has an extremely low vapor pressure, meaning it remains a non-volatile liquid residue.
This initial difference creates the first, most fundamental separation: the volatile metals are physically parted from the non-volatile ones.
The Three-Stage Separation Process
The genius of the vertical furnace lies in how it uses a carefully engineered temperature gradient to separate the metals in stages after they have been vaporized.
Stage 1: Vaporization in the Crucible
The process begins at the bottom of the furnace. The waste AZ91D alloy is placed in a crucible and heated to approximately 700°C.
The combination of high heat and low pressure causes the magnesium and zinc to sublimate or evaporate, forming a mixed metal vapor that begins to rise. The aluminum, along with other trace impurities, remains behind as a liquid.
Stage 2: Selective Condensation of Magnesium
As the mixed vapor of magnesium and zinc rises, it encounters a specifically cooled condensation disc. This disc is kept at a precise temperature that is cool enough for magnesium vapor to desublimate (turn directly from a gas to a solid).
However, this temperature is still too warm for the more volatile zinc to condense. As a result, high-purity magnesium crystals form and collect on this first disc.
Stage 3: Final Collection of Zinc
The remaining zinc vapor, having a higher vapor pressure, continues to travel past the magnesium collection zone.
It eventually reaches the coldest part of the furnace—the upper, water-cooled jacket. Here, the temperature is finally low enough to force the zinc vapor to desublimate into solid metal, completing the separation of all three components.
Understanding the Trade-offs
While elegant, this process is governed by a precise balance of physical parameters. Deviations can impact the quality and quantity of the recovered metals.
Purity vs. Yield
Achieving perfect separation requires extremely precise temperature control in the condensation zones. If the magnesium condensation disc is too cold, some zinc might co-condense with it, reducing the final purity of the magnesium. Conversely, if the disc is too warm, some magnesium vapor may fail to condense and pass by, reducing the overall yield.
Energy Consumption
Creating a high vacuum and heating a furnace to 700°C are both highly energy-intensive processes. The economic viability of the operation depends on balancing the cost of energy against the market value of the recovered pure metals.
Material Limitations
This method is exceptionally effective for alloys like AZ91D where the components have vast differences in vapor pressure. It would be far less effective, or entirely unsuitable, for separating metals with very similar volatility, as a clean separation via condensation would be nearly impossible.
Making the Right Choice for Your Goal
The operational focus of a vertical vacuum furnace can be tuned depending on the desired outcome.
- If your primary focus is recovering high-purity Magnesium: You must precisely control the temperature of the first condensation zone to ensure only magnesium desublimates.
- If your primary focus is simply removing Aluminum: The key is applying enough heat under vacuum to vaporize the volatile Mg/Zn mixture, leaving the aluminum behind as a residue.
- If your primary focus is maximizing overall efficiency: You must optimize the entire temperature gradient to balance the energy input with the yield and purity of all three separated metals.
By mastering these physical principles, a vertical vacuum furnace transforms complex waste alloy into three distinct, high-value streams of pure metal.
Summary Table:
| Separation Stage | Temperature | Key Action | Result |
|---|---|---|---|
| Stage 1: Vaporization | ~700°C | Mg & Zn vaporize under vacuum | Al remains as liquid residue |
| Stage 2: Mg Condensation | Cooled disc | Mg desublimates selectively | High-purity Mg crystals collected |
| Stage 3: Zn Condensation | Coldest zone (water-cooled) | Zn desublimates | Pure Zn collected separately |
Ready to recover high-purity metals from your alloy waste with precision?
Backed by expert R&D and manufacturing, KINTEK offers advanced Vacuum systems and other lab high-temp furnaces, all customizable for unique needs. Our vertical vacuum furnaces are engineered to maximize yield and purity for metals like magnesium, zinc, and aluminum.
Contact us today to discuss how our solutions can optimize your metal recovery process!
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What are the benefits of continuous sample movement in rotary tube furnaces? Boost Uniformity and Efficiency
- What are some applications of rotary tube furnaces? Ideal for Continuous High-Temperature Material Processing
- How is the structure of a rotary tube furnace characterized? Discover Its Key Components and Benefits
- What are the common applications of a rotary tube furnace? Achieve Uniform Heating for Powders and Granules
- What other fields utilize rotary tube furnaces? Discover Versatile Heating Solutions for Multiple Industries



















