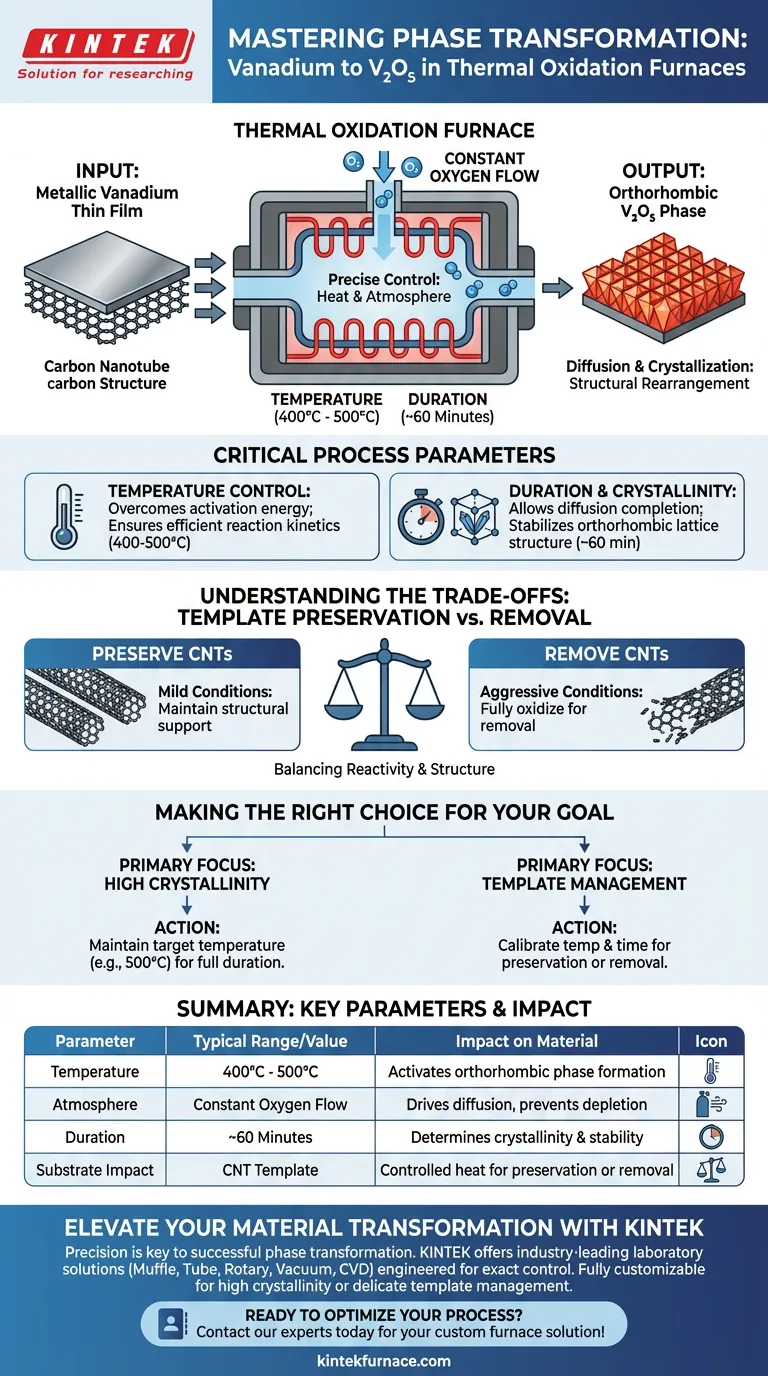
A thermal oxidation furnace facilitates the phase transformation of metallic vanadium into vanadium pentoxide (V2O5) by subjecting the material to a constant flow of oxygen at temperatures typically ranging from 400 °C to 500 °C. Through this high-temperature exposure, the metallic vanadium thin film undergoes diffusion and crystallization to structurally rearrange into the orthorhombic phase of V2O5.
The furnace acts as a critical control environment that governs not just the chemical oxidation, but the structural integrity of the final material. By precisely regulating heat and oxygen exposure, the process dictates the crystallinity of the V2O5 and the survival of the underlying carbon nanotube templates.

The Mechanism of Transformation
Establishing the Oxygen Atmosphere
The fundamental requirement for this transformation is a constant oxygen flow. The furnace ensures that the vanadium is continuously exposed to fresh reactant gas.
This prevents oxygen depletion at the surface of the metal. It drives the reaction forward effectively.
Diffusion and Crystallization
The transformation is not merely a surface reaction; it involves diffusion. Oxygen atoms penetrate the metallic vanadium structure while vanadium atoms migrate to form new bonds.
Simultaneously, the material undergoes crystallization. This rearranges the internal atomic structure from metallic vanadium into the specific orthorhombic crystal lattice of vanadium pentoxide.
Critical Process Parameters
Temperature Control
The furnace typically operates at precise set points, such as 400 °C or 500 °C. These specific thermal energy levels are required to overcome the activation energy barriers for phase transformation.
At these temperatures, the reaction kinetics are fast enough to occur efficiently. However, they are controlled enough to prevent unwanted degradation.
Duration and Crystallinity
The time the material spends in the furnace (e.g., 60 minutes) is a key variable. This duration allows the diffusion process to complete and the crystal structure to stabilize.
The length of exposure directly influences the crystallinity of the final product. A complete transformation requires sufficient time for the lattice to organize into the orthorhombic phase.
Understanding the Trade-offs
Template Preservation vs. Removal
A critical consideration when using a thermal oxidation furnace is the fate of the substrate. In this context, the vanadium is loaded onto carbon nanotubes (CNTs).
The furnace conditions create a trade-off regarding these templates. The specific combination of temperature and duration determines whether the CNTs are preserved as a structural support or removed during the process.
Balancing Reactivity and Structure
If the oxidation environment is too aggressive, you risk destroying the CNT template when preservation is desired. Conversely, if the conditions are too mild, the vanadium may not fully crystallize into the desired V2O5 phase.
Making the Right Choice for Your Goal
To achieve the desired material properties, you must tune the furnace parameters based on your specific objectives.
- If your primary focus is High Crystallinity: Ensure the furnace maintains the target temperature (e.g., 500 °C) for the full duration to maximize the formation of the orthorhombic phase.
- If your primary focus is Template Management: Carefully calibrate the temperature and time to either fully oxidize the carbon nanotubes for removal or maintain a lower threshold to preserve the nanotube structure.
By mastering the variables of heat and oxygen flow, you convert raw metallic film into a highly structured functional oxide.
Summary Table:
| Parameter | Typical Range/Value | Impact on Material |
|---|---|---|
| Temperature | 400 °C - 500 °C | Overcomes activation energy for orthorhombic phase formation. |
| Atmosphere | Constant Oxygen Flow | Drives diffusion and prevents oxygen depletion at the surface. |
| Duration | ~60 Minutes | Determines the degree of crystallinity and structural stability. |
| Substrate Impact | CNT Template | Controlled heat allows for either preservation or removal of CNTs. |
Elevate Your Material Transformation with KINTEK
Precision is the difference between a successful phase transformation and material degradation. KINTEK provides industry-leading laboratory solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered to provide the exact temperature control and atmosphere regulation required for complex processes like vanadium oxidation.
Backed by expert R&D and world-class manufacturing, our high-temp furnaces are fully customizable to meet your unique research or production needs. Whether you are aiming for high crystallinity V2O5 or delicate template management, KINTEK has the expertise to support your goals.
Ready to optimize your thermal processes?
Contact our experts today to find your custom furnace solution!
Visual Guide
References
- Matías Picuntureo, Samuel A. Hevia. The Synthesis of Sponge-like V2O5/CNT Hybrid Nanostructures Using Vertically Aligned CNTs as Templates. DOI: 10.3390/nano14020211
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What role does the vacuum or atmosphere control system play when processing TNZT alloys? Key to Biocompatible Purity
- Why is an Argon-Hydrogen gas mixture used in aerodynamic levitation? Achieve Pure Metal Melting and Precision Control
- What sealing features do box type atmosphere furnaces typically have? Essential for Precise Atmosphere Control
- Why is high-purity inert gas protection necessary in a laboratory furnace? Ensure Integrity for Sensitive Ceramics
- How does a box type atmosphere furnace achieve precise atmosphere control? Discover Key Systems for Reliable Heat Treatment
- What are the advantages of an atmosphere box furnace in the preparation and sintering of ceramic materials? Achieve Precise Control for Superior Ceramics
- What challenges are associated with inert atmosphere furnaces? Overcome High Costs and Complexity
- Why is a controlled oxygen environment necessary for high-entropy alloy powders? Master HEA Oxidation & Phase Purity



















