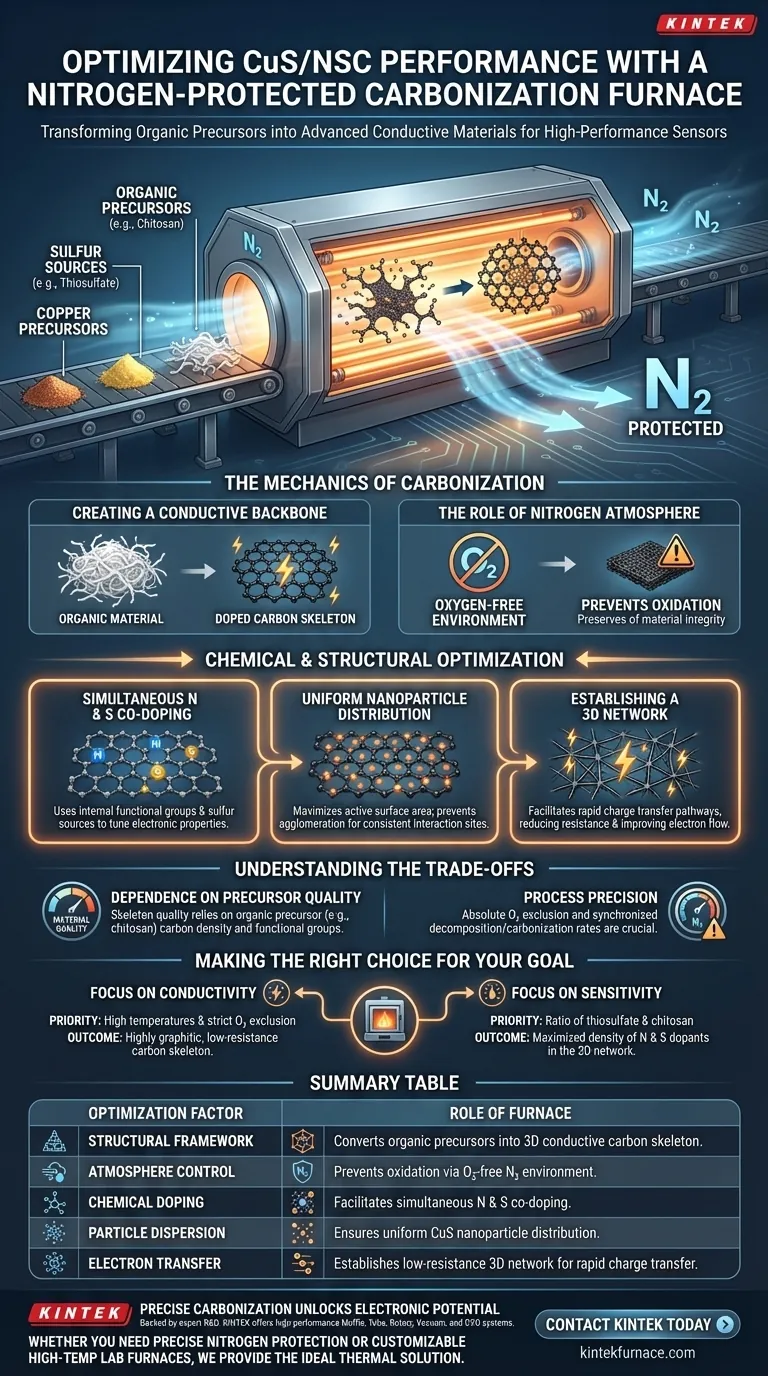
A nitrogen-protected carbonization furnace optimizes CuS/NSC performance by facilitating a high-temperature transformation that converts organic precursors into a highly conductive carbon framework without oxidation. This process simultaneously integrates nitrogen and sulfur dopants and ensures the uniform dispersion of copper sulfide nanoparticles, creating a robust 3D network essential for rapid electron transfer.
The furnace serves as a controlled reaction vessel that transforms insulating organic materials into a highly conductive, chemically doped skeleton. This structural and chemical evolution is critical for maximizing charge transfer speeds and overall sensor efficiency.

The Mechanics of Carbonization
Creating a Conductive Backbone
The primary function of the furnace is to process organic materials, such as chitosan, at high temperatures.
This thermal treatment converts the organic precursors into a doped carbon skeleton. This transformation changes the material from an insulator to a conductor, which is the fundamental requirement for electrochemical applications.
The Role of the Nitrogen Atmosphere
The process occurs in an oxygen-free environment protected by nitrogen gas.
This prevents the organic material from burning (oxidizing) at high temperatures. Instead, it forces the material to carbonize, preserving the structural integrity required for the final composite.
Chemical and Structural Optimization
Simultaneous N and S Co-doping
The furnace utilizes the precursor's internal functional groups as the source for nitrogen and carbon.
Simultaneously, it decomposes sulfur sources like thiosulfate. This results in the co-doping of the carbon lattice with nitrogen and sulfur, which tunes the electronic properties of the material for better performance.
Uniform Nanoparticle Distribution
The high-temperature processing ensures the uniform distribution of copper sulfide (CuS) nanoparticles within the carbon matrix.
By preventing particle agglomeration, the furnace maximizes the active surface area. This is crucial for maintaining consistent interaction sites throughout the material.
Establishing a 3D Network
The result of this process is a cohesive three-dimensional conductive network.
This 3D structure facilitates rapid charge transfer pathways. By reducing resistance and improving electron flow, the sensor performance is significantly enhanced compared to non-carbonized alternatives.
Understanding the Trade-offs
Dependence on Precursor Quality
The quality of the final conductive skeleton is entirely dependent on the specific organic precursors used (e.g., chitosan).
If the precursor lacks sufficient functional groups or carbon density, the resulting skeleton may be too fragile or insufficiently conductive for high-performance sensing.
Process Precision
The "oxygen-free" requirement is absolute; any leak in the nitrogen protection can lead to material degradation.
Furthermore, the decomposition rates of the sulfur source must align with the carbonization rate of the organic material. Mismatches here can lead to uneven doping or poor structural integration.
Making the Right Choice for Your Goal
To maximize the potential of CuS/NSC materials, consider how you manage the carbonization parameters:
- If your primary focus is Conductivity: Prioritize high temperatures and strict oxygen exclusion to ensure the formation of a highly graphitic, low-resistance carbon skeleton.
- If your primary focus is Sensitivity: Focus on the ratio of thiosulfate and chitosan to maximize the density of nitrogen and sulfur dopants within the 3D network.
The carbonization furnace is not just a heat source; it is the tool that defines the electronic and structural DNA of your final sensor material.
Summary Table:
| Optimization Factor | Role of Nitrogen-Protected Furnace |
|---|---|
| Structural Framework | Converts organic precursors (e.g., chitosan) into a 3D conductive carbon skeleton. |
| Atmosphere Control | Prevents oxidation via an oxygen-free nitrogen environment to preserve material integrity. |
| Chemical Doping | Facilitates simultaneous N and S co-doping to tune electronic properties. |
| Particle Dispersion | Ensures uniform CuS nanoparticle distribution to maximize active surface area. |
| Electron Transfer | Establishes a low-resistance 3D network for rapid charge transfer. |
Precision carbonization is the key to unlocking the electronic potential of advanced materials like CuS/NSC. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of material science. Whether you need precise nitrogen protection or customizable high-temp lab furnaces for your unique research needs, our team is ready to provide the ideal thermal solution. Contact KINTEK today to optimize your material performance!
Visual Guide
References
- Haibing Zhu, Zhanjun Yang. Non-Enzymatic Electrochemical Glucose Sensors Based on Metal Oxides and Sulfides: Recent Progress and Perspectives. DOI: 10.3390/chemosensors13010019
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why are vacuum or atmosphere control systems required for Fe, Co, and Ni single-atom catalysts? Ensure Atomic Precision
- Why is a tube furnace or box furnace with atmosphere control required for debinding SiC? Ensure Structural Integrity
- Why is precise temperature control at 500 °C necessary in a Pyrolysis Furnace? Maximize Carbon Fiber Recovery
- Why is an oxidizing atmosphere necessary for NCM90 solid-state synthesis? Control Nickel Oxidation and Lattice Purity
- What are some related terms associated with atmosphere furnaces? Explore Types for Your Heat Treatment Needs
- Why is a sealed environment important in a controlled atmosphere furnace? Ensure Precision and Safety in High-Temp Processes
- Why is a high-temperature atmosphere tube furnace required for the synthesis of Sr2TiO4-NF through ammonolysis?
- Which methanol dissociation reaction is appropriate for carburizing or neutral hardening? Ensure Clean, Controlled Heat Treatment



















