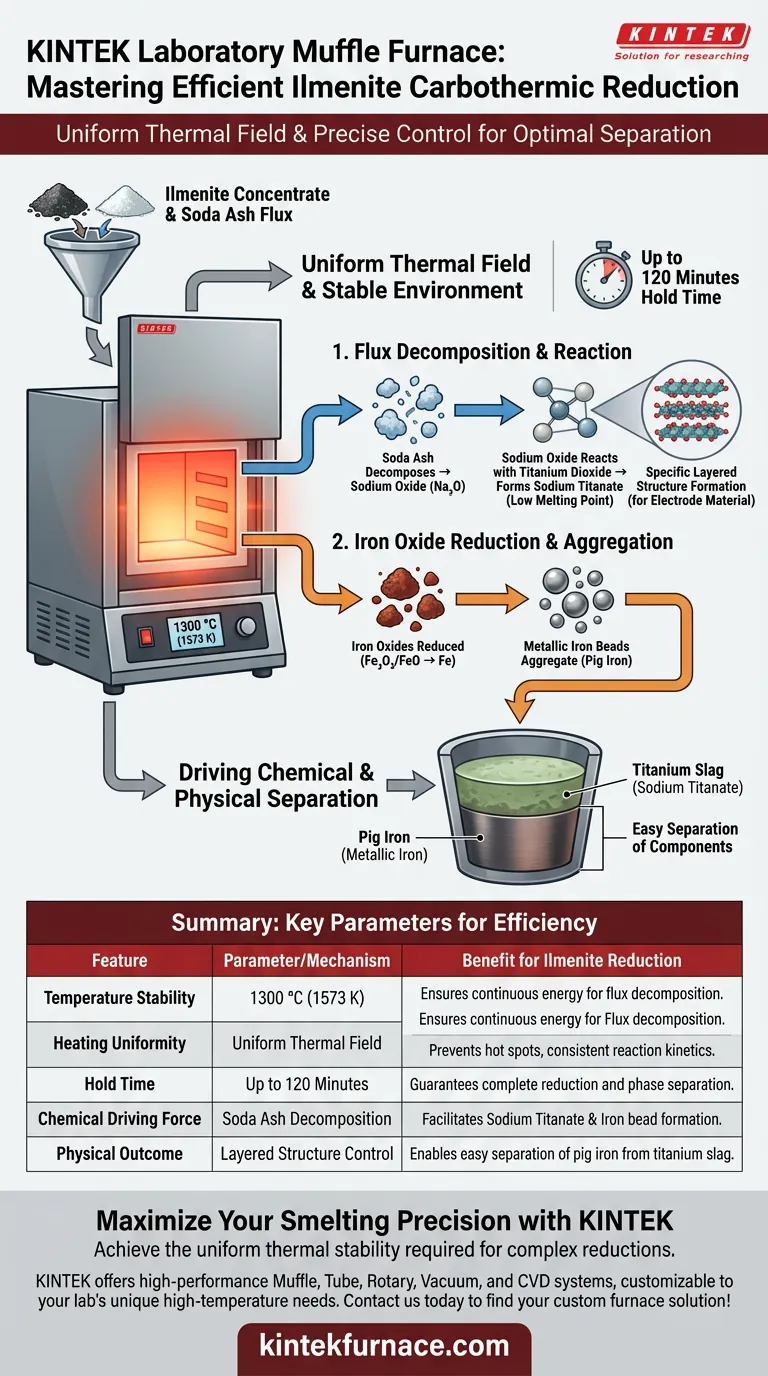
A laboratory muffle furnace maintains efficiency primarily by generating a uniform thermal field at 1300 °C (approx. 1573 K). This precise heat distribution ensures the continuous energy supply needed to decompose fluxes and drive the chemical transformation of ilmenite concentrate into separable iron and titanium components.
By providing a stable high-temperature environment for up to 120 minutes, the furnace enables the simultaneous decomposition of soda ash and reduction of iron oxides, ultimately forcing the physical separation of metallic iron beads from the titanium slag.

The Mechanism of Thermal Stability
To understand the efficiency of the carbothermic reduction, you must look beyond simple heating. The core function of the muffle furnace is to provide a consistent energy baseline that drives specific endothermic reactions.
Uniform Energy Distribution
The furnace chamber creates a uniform thermal field around the sample pellets.
Unlike direct heating methods that might create hot spots, the muffle furnace envelopes the sample in consistent heat. This uniformity is critical for maintaining the energy required for the reaction throughout the entire sample volume, not just the surface.
Flux Decomposition
The stable 1300 °C environment triggers the decomposition of the soda ash flux.
As the thermal energy penetrates the pellets, the soda ash breaks down to produce sodium oxide. This is the chemical precursor required to alter the properties of the titanium within the concentrate.
Driving Chemical and Physical Separation
The deep need in this process is separating the valuable titanium component from the iron. The furnace’s environment facilitates this by chemically altering the melting points of the constituents.
Formation of Sodium Titanate
The sodium oxide produced by the decomposed flux reacts with titanium dioxide in the ilmenite.
This reaction forms sodium titanate, a compound with a significantly lower melting point than the surrounding materials. This chemical shift is essential for creating a distinct slag phase that can be separated later.
Reduction of Iron Oxides
Simultaneously, the high-temperature environment promotes the reduction of iron oxides found in the ilmenite.
Because the furnace maintains this temperature explicitly (often between 1573 K and 1673 K), the reaction kinetics are fast enough to fully reduce the oxides.
Aggregation of Metallic Iron
The final stage of this efficient environment is the aggregation of metallic iron beads.
Because the thermal field is stable, the reduced iron is able to coalesce into distinct metallic beads. This physical aggregation allows for the efficient separation of the pig iron from the titanium slag.
Understanding the Operational Requirements
While the muffle furnace provides the ideal environment, efficiency relies on precise parameter control. The process is not instantaneous; it requires maintaining these conditions over a set duration.
Temperature and Time Constraints
The supplementary data indicates that maintaining temperatures between 1573 K and 1673 K is necessary for optimal conversion.
Furthermore, this environment must be held for a specific duration, such as 120 minutes. Deviating from this timeframe can result in incomplete reduction or insufficient phase separation, rendering the process inefficient.
Layered Structure Formation
Proper thermal control does more than just melt components; it dictates the crystal structure.
The sustained heat directly induces the formation of sodium-based titanate phases with specific layered structures. If the furnace environment fluctuates, these specific structural properties may not form correctly, affecting the quality of the final electrode material.
Making the Right Choice for Your Goal
The muffle furnace is a tool for precision. Depending on your specific research or production goals, you should prioritize different aspects of its operation.
- If your primary focus is Purity of Separation: Prioritize the stability of the thermal field at 1300 °C to ensure distinct iron bead aggregation and clean slag formation.
- If your primary focus is Material Structure: Focus on the duration of the heat treatment (e.g., 120 minutes) to guarantee the complete formation of the specific layered sodium titanate structures.
Efficiency in carbothermic reduction is not just about reaching a high temperature; it is about maintaining a uniform thermal baseline that allows chemical decomposition and physical separation to occur in tandem.
Summary Table:
| Feature | Parameter/Mechanism | Benefit for Ilmenite Reduction |
|---|---|---|
| Temperature Stability | 1300 °C (1573 K) | Ensures continuous energy for flux decomposition. |
| Heating Uniformity | Uniform Thermal Field | Prevents hot spots, ensuring consistent reaction kinetics. |
| Hold Time | Up to 120 Minutes | Guarantees complete iron oxide reduction and phase separation. |
| Chemical Driving Force | Soda Ash Decomposition | Facilitates sodium titanate formation and iron bead aggregation. |
| Physical Outcome | Layered Structure Control | Enables easy separation of pig iron from titanium slag. |
Maximize Your Smelting Precision with KINTEK
Achieve the uniform thermal stability required for complex carbothermic reductions and material synthesis. Backed by expert R&D and precision manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to your laboratory’s unique high-temperature needs. Whether you are refining ilmenite concentrate or developing advanced electrode materials, our furnaces provide the 1300°C consistency your research demands.
Ready to elevate your lab’s efficiency? Contact us today to find your custom furnace solution!
Visual Guide
References
- Efficiency of Soda-Technology Carbothermal Smelting of Thermoactivated Ilmenite Concentrate with Aluminosilicate Mineralization. DOI: 10.3390/min15090906
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What should be considered regarding the controller when purchasing a muffle furnace? Choose the Right Controller for Precision
- What are the environmental concerns associated with traditional crucible furnaces? High Emissions & Low Efficiency Explained
- What role does a high-temperature box resistance furnace play in Hydroxyapatite/Zirconia composite preparation?
- Can a muffle furnace be used for pyrolysis? Unlock Precise Thermal Decomposition
- What is a muffle furnace and what is its primary use? Ensure Purity in High-Temperature Processes
- What role do muffle furnaces play in biomedical applications? Essential for Purity and Precision in Medical Research
- How does the muffle furnace ensure uniform heating? Achieve Precise, Even Heat for Your Lab
- What is the purpose of using a muffle furnace to fire Al2O3 ceramic shells at 1050°C? Enhance Strength and Purity



















