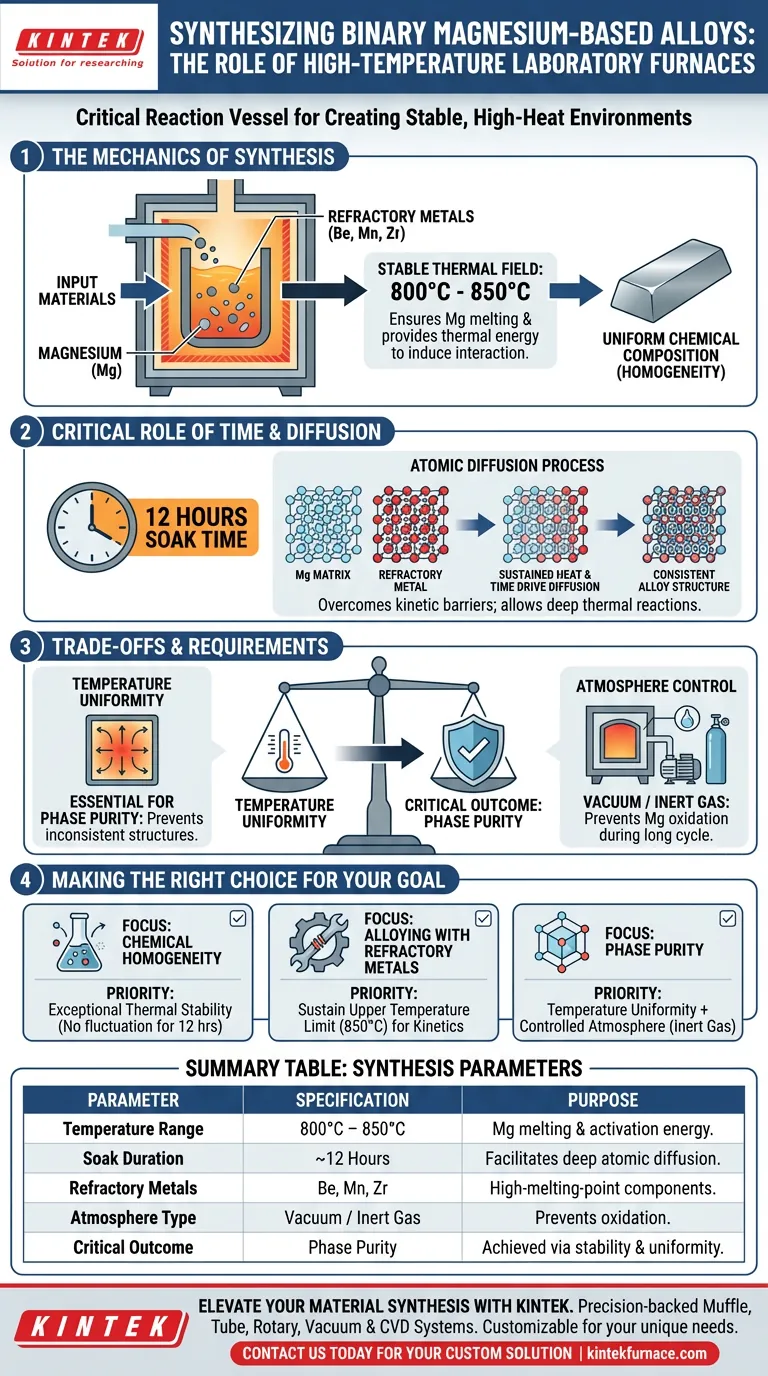
A high-temperature laboratory furnace serves as the critical reaction vessel for synthesizing binary magnesium-based alloys by creating a highly stable, high-heat environment. It facilitates the complete melting and fusion of magnesium with refractory metals—specifically beryllium, manganese, and zirconium—by maintaining temperatures between 800°C and 850°C for extended periods, typically around 12 hours.
The furnace’s primary role is to overcome kinetic barriers between magnesium and high-melting-point refractory components. Through sustained, uniform heating, it ensures sufficient atomic diffusion, resulting in binary alloy samples with a consistent chemical composition.

The Mechanics of Magnesium Alloy Synthesis
Establishing a Stable Thermal Field
The fundamental requirement for this synthesis is a consistent thermal field. The furnace must reach and maintain a temperature range of 800°C to 850°C.
This specific range is selected to ensure the magnesium enters a molten state while providing enough thermal energy to induce interaction with the refractory metals.
Facilitating Fusion with Refractory Metals
Magnesium is often alloyed with metals that have significantly higher melting points or different densities, such as beryllium, manganese, and zirconium.
The furnace provides the necessary "melting kinetic environment." This allows the lower-melting-point magnesium to dissolve or react with these harder-to-melt refractory components, initiating the fusion process.
Ensuring Uniform Chemical Composition
The ultimate goal of using a high-precision furnace is to achieve homogeneity. Without a stable thermal environment, the alloy could suffer from segregation, where elements separate rather than mix.
By controlling the heat input precisely, the furnace ensures that the resulting sample has a uniform chemical makeup throughout the ingot.
The Critical Role of Time and Diffusion
Driving Diffusion Through Duration
Heat alone is often insufficient for perfect alloying; time is the second critical variable. The process requires a sustained "soak" time of approximately 12 hours.
This extended duration allows for deep thermal reactions. It gives the atoms of the refractory metals sufficient time to diffuse evenly through the magnesium matrix.
Overcoming Kinetic Inertia
Solid-state or mixed-phase reactions can be sluggish. The 12-hour hold time at high temperatures provides the activation energy needed to break stable bonds in raw materials and form new, stable chemical bonds between the differing elements.
Understanding the Trade-offs and Requirements
Temperature Uniformity vs. Phase Purity
A critical trade-off in furnace selection is the balance between raw heating power and thermal uniformity.
As noted in broader synthesis contexts, a high degree of temperature uniformity is essential for phase purity. Fluctuations in temperature across the furnace zone can lead to incomplete crystal structures or inconsistent alloy phases.
Atmosphere Control
While the primary mechanism is thermal, the environment inside the furnace is equally important. Magnesium is highly reactive to oxygen.
Although the core heating profile is 800-850°C, advanced laboratory furnaces (such as tube or box furnaces) often employ vacuum or inert gas atmospheres. This prevents oxidation during the long 12-hour heating cycle, ensuring the structural integrity of the final alloy.
Making the Right Choice for Your Goal
To achieve the best results in magnesium alloy synthesis, align your process parameters with your specific metallurgical objectives:
- If your primary focus is Chemical Homogeneity: Prioritize a furnace with exceptional thermal stability to maintain the 800-850°C range without fluctuation for the full 12-hour cycle.
- If your primary focus is Alloying with Refractory Metals: Ensure the furnace can sustain the upper limit of the temperature range (850°C) to maximize the diffusion kinetics of elements like Zirconium.
- If your primary focus is Phase Purity: Select a furnace setup that combines temperature uniformity with a controlled atmosphere (inert gas) to prevent oxide contamination during the long fusion process.
Success in synthesizing binary magnesium alloys relies on the precise intersection of sufficient heat, extended time, and absolute thermal stability.
Summary Table:
| Parameter | Specification | Purpose in Synthesis |
|---|---|---|
| Temperature Range | 800°C – 850°C | Ensures Mg melting & provides activation energy for refractory metals. |
| Soak Duration | ~12 Hours | Facilitates deep atomic diffusion for consistent chemical composition. |
| Refractory Metals | Be, Mn, Zr | High-melting-point components that require sustained heat to fuse with Mg. |
| Atmosphere Type | Vacuum / Inert Gas | Essential to prevent highly reactive magnesium from oxidizing during heating. |
| Critical Outcome | Phase Purity | Achieved through temperature uniformity and stable thermal fields. |
Elevate Your Material Synthesis with KINTEK
Precision is the backbone of metallurgy. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temperature furnaces—all fully customizable to meet your unique alloy synthesis needs.
Whether you are fusing refractory metals or ensuring chemical homogeneity in magnesium-based samples, our systems provide the thermal stability and atmospheric control required for success.
Ready to optimize your research? Contact us today to find your custom furnace solution!
Visual Guide
References
- В. Н. Володин, Xeniya Linnik. Recycling of beryllium, manganese, and zirconium from secondary alloys by magnesium distillation in vacuum. DOI: 10.31643/2024/6445.42
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What safety features are incorporated in muffle furnace designs? Ensure Operator and Equipment Protection
- What is the purpose of ashing in a muffle furnace? Unlock Material Purity and Quality Insights
- What are the construction details of a typical muffle furnace? Key Components for High-Temperature Control
- What is the reputation of box furnaces in terms of quality and reliability? Trusted for Decades in High-Stakes Applications
- How do sample requirements influence the choice of a muffle furnace? Match Your Material for Accurate Results
- What temperature considerations are important for muffle furnaces? Optimize Performance and Longevity
- Why is the type of controller important in a muffle furnace? Unlock Precision and Repeatability for Your Lab
- What factors affect the price range of muffle furnaces? Key Drivers for Smart Lab Investment



















