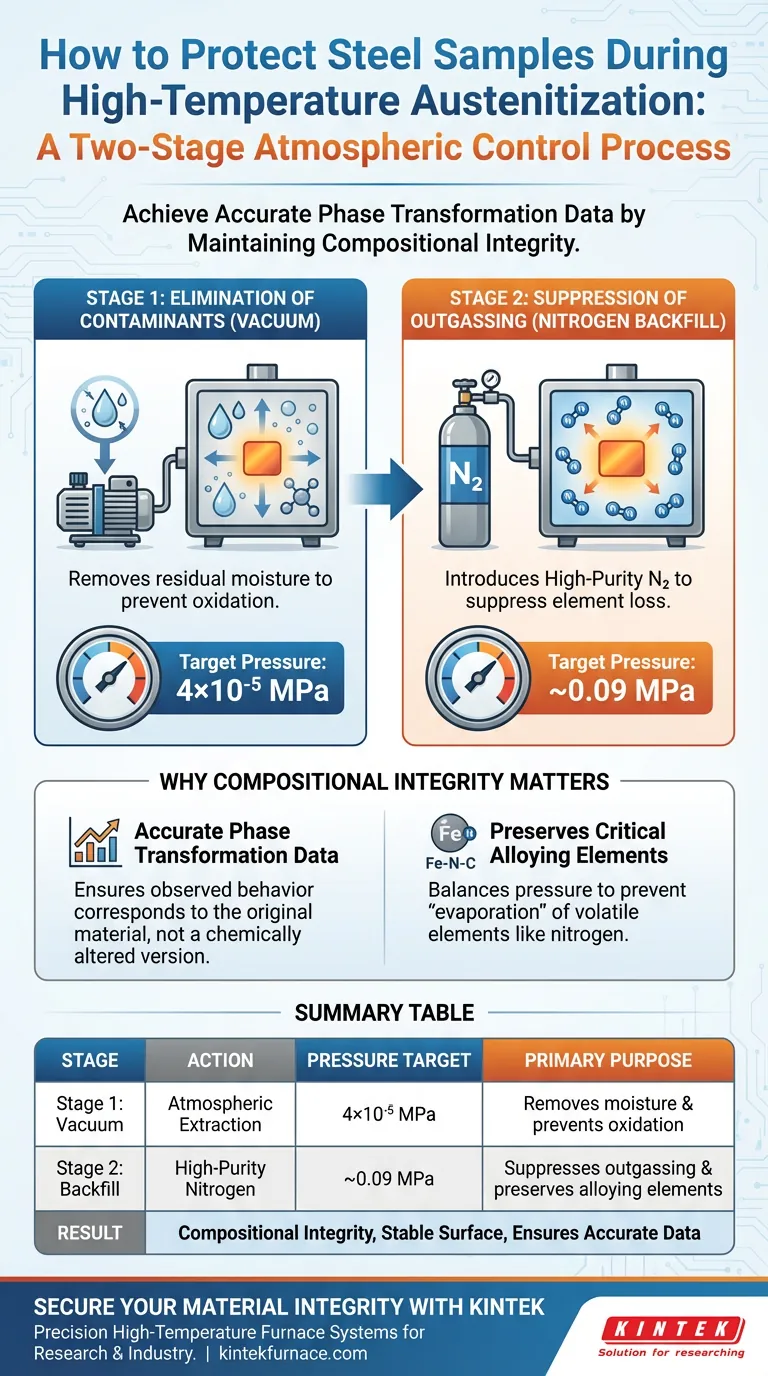
The protection of steel samples during high-temperature austenitization relies on a precise two-step sequence of atmospheric control. First, a vacuum pump extracts the atmosphere to remove environmental contaminants, specifically residual moisture. Second, high-purity nitrogen gas is introduced to create a protective, pressurized environment that stabilizes the sample's surface and chemical composition.
Core Takeaway Achieving accurate phase transformation data requires maintaining the steel's exact original composition throughout the heating cycle. This method prevents the "evaporation" of alloying elements by balancing the internal pressure of the material with an external nitrogen atmosphere.

The Two-Stage Protection Mechanism
To understand how this process works, we must look at the distinct roles played by the vacuum state and the nitrogen backfill.
Stage 1: Elimination of Contaminants
The process begins by using a vacuum pump to drastically reduce the chamber pressure.
The target pressure is typically lowered to approximately 4×10⁻⁵ MPa.
This deep vacuum is critical for removing residual moisture and air from the environment, which are primary sources of oxidation and contamination.
Stage 2: Suppression of Outgassing
Once the moisture is removed, high-purity nitrogen gas is introduced into the chamber.
The pressure is raised to roughly 0.09 MPa (slightly below standard atmospheric pressure).
This establishes a controlled environment that physically suppresses "outgassing," a phenomenon where gases trapped inside the metal or volatile elements on the surface attempt to escape at high temperatures.
Preserving Critical Alloying Elements
For certain steel grades, preserving the nitrogen content within the alloy is vital.
Without the external pressure of the high-purity nitrogen gas, the steel would lose its native nitrogen to the atmosphere.
By balancing the pressure, this method prevents the loss of these alloying elements, ensuring the material retains its intended chemical identity.
Why Compositional Integrity Matters
The ultimate goal of this protection method is data fidelity.
Ensuring Accurate Phase Transformation Data
Austenitization is often performed to study how the steel's structure changes (phase transformation).
If the chemical composition changes during heating—due to moisture contamination or the loss of nitrogen—the resulting data will be flawed.
The vacuum-then-nitrogen protocol ensures that the phase transformation behavior observed corresponds exactly to the original material composition, not a chemically altered version of it.
Common Pitfalls to Avoid
While this process is robust, it relies on strict adherence to the pressure parameters.
Inadequate Vacuum Levels
Failing to reach the initial low pressure (4×10⁻⁵ MPa) leaves residual moisture in the furnace.
This moisture acts as a contaminant, reacting with the steel surface even after nitrogen is introduced, potentially skewing results.
Incorrect Nitrogen Pressure
If the nitrogen backfill pressure is too low, it may not sufficiently suppress outgassing.
Conversely, while not explicitly detailed in the primary data for steel, using the wrong gas type or purity level could introduce new impurities rather than protecting the sample.
Making the Right Choice for Your Goal
To apply this technical insight to your own high-temperature processes, consider your specific objectives.
- If your primary focus is preserving complex alloy compositions: Ensure you backfill with nitrogen to ~0.09 MPa to mechanically suppress the loss of volatile elements like nitrogen.
- If your primary focus is eliminating surface oxidation: Prioritize the initial vacuum stage to reach at least 4×10⁻⁵ MPa to guarantee the total removal of residual moisture.
Success in high-temperature analysis is defined not just by the heat applied, but by the purity of the environment maintained.
Summary Table:
| Stage | Action | Pressure Target | Primary Purpose |
|---|---|---|---|
| Stage 1: Vacuum | Atmospheric Extraction | 4×10⁻⁵ MPa | Removes moisture and prevents oxidation |
| Stage 2: Backfill | High-Purity Nitrogen | ~0.09 MPa | Suppresses outgassing and preserves alloying elements |
| Result | Compositional Integrity | Stable Surface | Ensures accurate phase transformation data |
Secure Your Material Integrity with KINTEK
Don’t let atmospheric contamination or element loss compromise your research. KINTEK’s high-temperature furnace systems—including our specialized Vacuum and CVD solutions—are engineered for precision. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, and Rotary furnaces designed to meet the rigorous demands of steel austenitization and material science.
Ready to elevate your lab’s heating precision? Contact us today to find your custom solution!
Visual Guide
References
- Philip König, Sebastian Weber. Isothermal Bainitic Transformation in High-Alloyed C + N Steel: Influence of Carbon and Nitrogen on Microstructure and Mechanical Properties. DOI: 10.1007/s11661-025-07851-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is a vacuum heat treatment furnace and what technology does it combine? Unlock Purity and Precision in Heat Treatment
- What are the key specifications of vacuum carburizing furnaces? Optimize Your Heat Treatment Process
- What are the different charge operation methods for multi-chamber furnaces? Optimize Your Heat Treatment Process
- How are vacuum furnaces used in metal heat treatment? Enhance Metal Quality with Precision Heat Processing
- What role does rotary mechanical stirring play in the high-temperature synthesis of mesophase pitch? Maximize Homogeneity
- Why is a vacuum furnace beneficial for applications requiring high purity? Achieve Unmatched Material Purity and Performance
- How does a vacuum furnace prevent oxidation of metals? Unlock Purity and Strength in Heat Treatment
- What are the advantages of using a vacuum heat treatment furnace? Achieve Superior Material Quality and Control



















