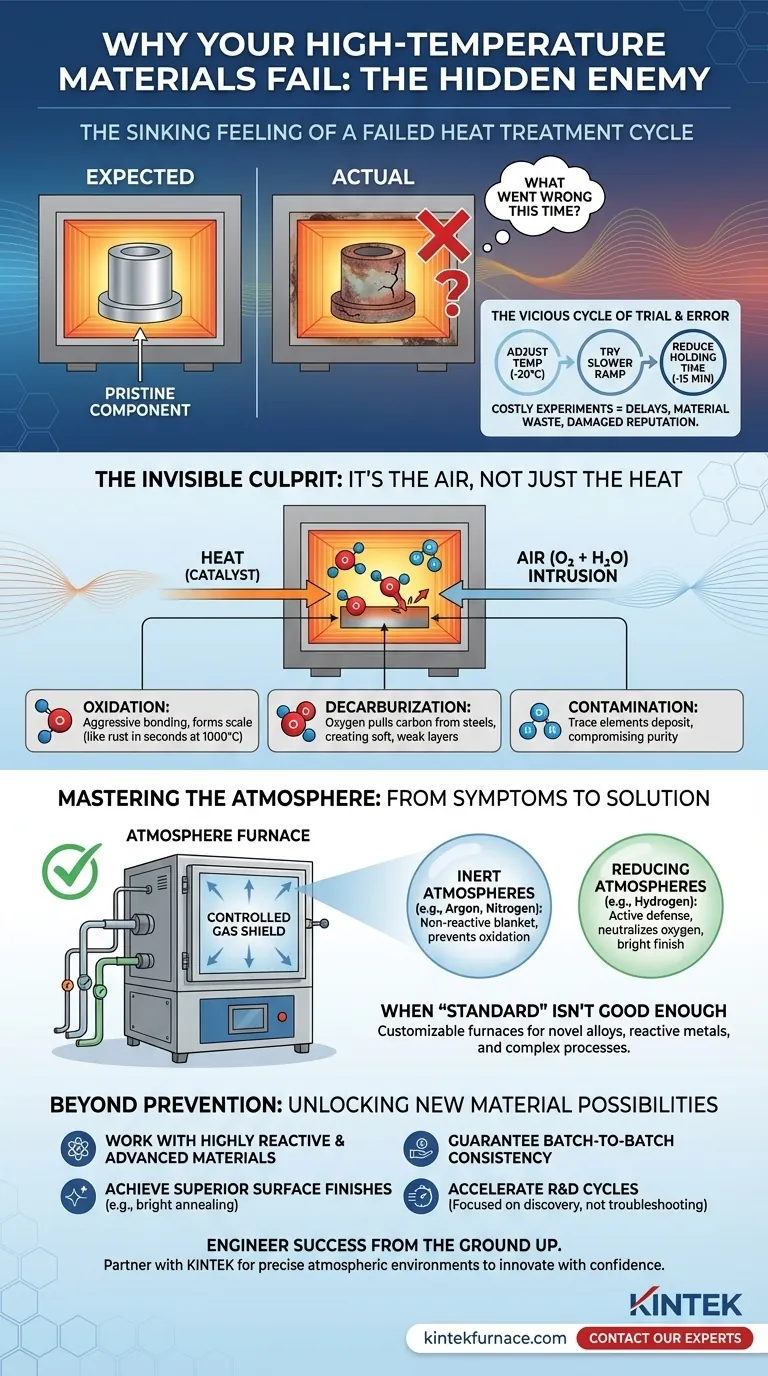
The Sinking Feeling of a Failed Heat Treatment Cycle
You open the furnace door after a critical, multi-hour heating cycle. Inside should be a pristine, perfectly treated component. Instead, your heart sinks. The surface is discolored, covered in a brittle, flaky scale, or its properties have fundamentally changed for the worse.
Weeks of research, valuable materials, and significant energy costs are compromised in an instant. You're left staring at a failed part, asking the frustrating question: what went wrong this time?
The Vicious Cycle of Trial and Error
If this scenario feels familiar, you are not alone. It's a pervasive problem in materials science, metallurgy, and advanced manufacturing. The immediate instinct is to blame the most obvious variable: the heat.
So begins the frustrating guesswork:
- "Maybe the temperature was too high? Let's lower it by 20 degrees."
- "Perhaps the ramp rate was too fast? We'll try a slower profile."
- "Was the holding time too long? Let's cut it by 15 minutes."
Each attempt is another costly, time-consuming experiment that often leads to the same inconsistent results. This isn't just a technical nuisance; it's a serious business obstacle. It creates project delays that push back product launches, causes material waste that inflates R&D budgets, and produces unreliable components that can damage a company's reputation for quality. You're stuck troubleshooting a fundamental process instead of advancing your research or production.
The Invisible Culprit: It's Not the Heat, It's the Air
Here is the fundamental truth that breaks the cycle: The primary cause of failure is rarely the temperature itself, but the uncontrolled chemical reactions that heat accelerates. The real enemy is invisible: the ordinary air filling your furnace chamber.
Heat acts as a powerful catalyst. At high temperatures, the oxygen and water vapor in the air, which are harmless at room temperature, become incredibly aggressive. They actively attack the surface of your material.
- Oxidation: Oxygen aggressively bonds with metals and ceramics, forming the unwanted scale and discoloration you see on failed parts. A process like iron rusting, which takes years in your backyard, can happen in seconds at 1000°C.
- Decarburization: For steels, the oxygen in the air can literally pull carbon atoms out of the material's surface, creating a soft, weak outer layer.
- Contamination: Trace elements in the air can deposit onto your material, compromising its purity and performance in sensitive applications like semiconductors or medical implants.
This is why simply tweaking temperature profiles is like trying to fix a leak by painting over the water stain. You are only addressing the symptom, not the root cause of the problem.
From Fighting Symptoms to Curing the Disease: Mastering the Atmosphere
If the root problem is unwanted reactions with air, the logical solution is to get rid of the air. To achieve perfect, repeatable results, you must replace the unpredictable, reactive environment of air with a controlled, predictable, and protective one.
This is the entire purpose of a specialized Atmosphere Furnace. It's a tool designed not just to heat a material, but to give you absolute control over the chemical environment surrounding it.
Instead of leaving your material exposed, you create a protective shield using a specific gas:
- Inert Atmospheres (e.g., Argon, Nitrogen): These gases act like a perfect, non-reactive blanket. They physically displace all the oxygen, preventing any oxidation from ever starting. Your material is heated in a neutral bubble, emerging just as pure as it went in.
- Reducing Atmospheres (e.g., Hydrogen): These go a step further. They are an active defense, seeking out and reacting with any stray oxygen molecules to neutralize them. A reducing atmosphere can even reverse light surface oxidation, resulting in a bright, clean finish.
A properly designed atmosphere furnace, with its sealed chamber and precise gas delivery system, solves the root cause. It transforms heat treatment from a game of chance into a predictable science.
When 'Standard' Isn't Good Enough
But what if you're working with a novel alloy, a highly reactive metal like titanium, or a complex multi-stage process? An off-the-shelf furnace might not provide the granular control you need. This is where the ability to precisely define the environment becomes critical. True process control requires a furnace built for your specific challenge—one that can handle the exact gas mixture, purity levels, and pressure cycles your material demands.
Beyond Prevention: Unlocking New Material Possibilities
Once you stop fighting unpredictable failures, you can start achieving intentional breakthroughs. Mastering the furnace atmosphere isn't just about damage control; it's about unlocking potential.
When your process is stable, repeatable, and precisely controlled, you can:
- Work with highly reactive and advanced materials that are impossible to process in open air.
- Achieve superior surface finishes, like bright annealing, without costly and time-consuming secondary cleaning steps.
- Guarantee batch-to-batch consistency, moving your innovation from a laboratory curiosity to a reliable, manufacturable product.
- Accelerate R&D cycles because you are focused on genuine discovery, not on re-running failed basic experiments.
You are no longer just heating a material; you are performing precise surface engineering, opening the door to creating materials with properties that were previously out of reach.
Your material challenges are unique, and your furnace should be too. Stop wrestling with unpredictable results and start engineering success from the ground up. At KINTEK, our deep customization capabilities mean we don't just sell you a furnace; we partner with you to design the precise atmospheric environment you need to innovate with confidence. Let's discuss the specific requirements of your project. Contact Our Experts to begin the conversation.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
Related Articles
- More Than a Box: The Engineering Philosophy Behind High-Integrity Vacuum Furnaces
- Why Your High-Temp Experiments Fail: It’s Not the Heat, It’s the Atmosphere
- In Pursuit of Purity: The Silent Power of the Vacuum Furnace
- The Invisible Contaminant: Why Your Furnace Atmosphere is Sabotaging Your Results
- The Engineer's Gambit: Why Vacuum Furnaces Are About Control, Not Just Heat




















