You’ve run a flawless heating cycle. The pressure gauge confirms a deep, stable vacuum. You followed the procedure to the letter. But when you finally open the furnace chamber, your heart sinks. The high-purity metal—which should be pristine and bright—is tarnished, discolored, and oxidized. The entire batch, and the hours of work it represents, is a write-off. What went wrong?
The Vicious Cycle of Troubleshooting the Wrong Problem
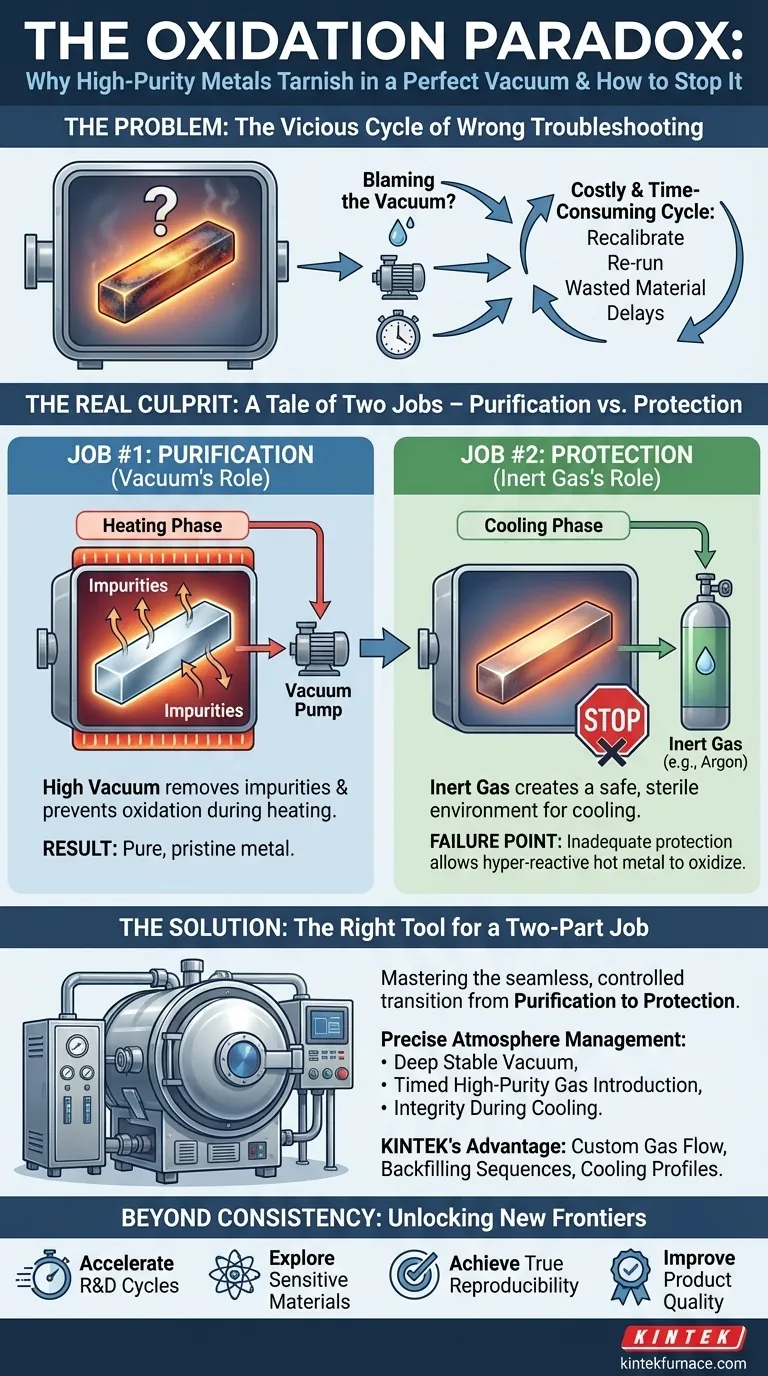
If this scenario sounds painfully familiar, you're not alone. In laboratories and R&D centers worldwide, this exact failure is a recurring source of frustration. The immediate, and logical, reaction is to blame the vacuum.
Engineers and researchers instinctively start troubleshooting the purification phase:
- "Is there a microscopic leak in the chamber?"
- "Did the vacuum pump underperform?"
- "Should we increase the hold time at temperature?"
This leads to a costly and time-consuming cycle of recalibrating equipment, re-running tests, and scrutinizing the vacuum system—all while the true cause remains hidden.
The business consequences are significant. Each failed batch translates directly into wasted high-value materials, critical project delays, and a loss of confidence in R&D outcomes. When results are inconsistent, it becomes impossible to reliably develop new materials or guarantee the quality of existing ones, putting innovation and production timelines at risk.
The Real Culprit: A Tale of Two Jobs—Purification vs. Protection
The turning point comes when we realize that the problem isn't happening during the purification step. It's happening after. The persistent oxidation is not a failure of the vacuum; it's a failure of protection during the critical cooling phase.
A vacuum furnace process has two distinct, equally important jobs:
Job #1: Purification (The Vacuum's Role)
Under high vacuum, volatile impurities and dissolved gases are boiled off and pumped away. The near-total absence of oxygen prevents the metal from oxidizing as it heats up. The vacuum does this job exceptionally well, creating a metal that is chemically pure and pristine.
Job #2: Protection (The Inert Gas's Role)
Here's the paradox: the success of the vacuum creates a new vulnerability. At the end of the heating cycle, you have a perfectly pure metal that is extremely hot. In this state, it is hyper-reactive. If you were to let ambient air into the chamber, it would oxidize instantly, undoing all your hard work.
This is where the inert gas, like argon, comes in. Its job is not to clean the metal—that’s already done. Its job is to act as a bodyguard. By backfilling the chamber with a high-purity, non-reactive gas, you create a safe, sterile environment for the metal to cool down without being exposed to oxygen.
The "common solutions" of tweaking the vacuum fail because they are addressing the wrong job. It's like blaming the surgeon for a post-op infection that happened because the recovery room wasn't sterile. Your purification was perfect; the failure was in safeguarding the result.
The Right Tool for a Two-Part Job
To solve this problem permanently, you don't need a more powerful vacuum. You need a furnace system that masters the seamless, controlled transition from purification to protection.
This requires more than just a chamber that can hold a vacuum; it requires precise control over the entire atmosphere management cycle. You need a system that can:
- Maintain a deep, stable vacuum to guarantee purification.
- Introduce a high-purity inert gas at the exact right time and pressure.
- Ensure the integrity of the atmosphere during the entire cooling phase, preventing any contaminants from entering.
This is precisely why KINTEK's Vacuum & Atmosphere Furnaces are engineered to excel. We understand that creating a pure material and preserving it are two sides of the same coin. Our furnaces are designed not just to achieve an excellent vacuum, but to provide the sophisticated gas control systems and chamber integrity needed to flawlessly manage the critical handoff from a vacuum environment to a protective inert atmosphere. Our deep customization capabilities mean we can tailor gas flow, backfilling sequences, and cooling profiles to the specific needs of your highly reactive materials.
Beyond Consistency: Unlocking New Material Frontiers
When you can finally trust your furnace to deliver a perfect, non-oxidized product every single time, the focus shifts from troubleshooting to innovation. The energy once spent on re-running failed experiments is now freed up for true progress.
With a reliable process, you can:
- Accelerate R&D Cycles: Move confidently from one experiment to the next, knowing your results are not being compromised by process flaws.
- Explore More Sensitive Materials: Work with advanced alloys and reactive metals that were previously too challenging to handle, opening up new avenues for discovery.
- Achieve True Reproducibility: Generate the consistent, high-quality data needed for academic publication or scaling up to pilot production.
- Improve Product Quality: Ensure that the materials you develop and produce meet the highest standards of purity and performance, every time.
Your material challenges are unique. Our ability to solve them is, too. Let's move beyond troubleshooting and start innovating. To discuss how a precisely controlled furnace atmosphere can safeguard your most critical projects, Contact Our Experts.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
Related Articles
- The Unseen Advantage: How Vacuum Furnaces Forge Metallurgical Perfection
- Why Your Vacuum Furnace Failed After the Lab Move—And How to Prevent It
- The Pursuit of Nothing: How Vacuum Furnace Control Defines Material Destiny
- The Physics of Perfection: Deconstructing Temperature Control in a Vacuum Furnace
- Beyond the Void: The Hidden Costs of a Perfect Vacuum Furnace




















