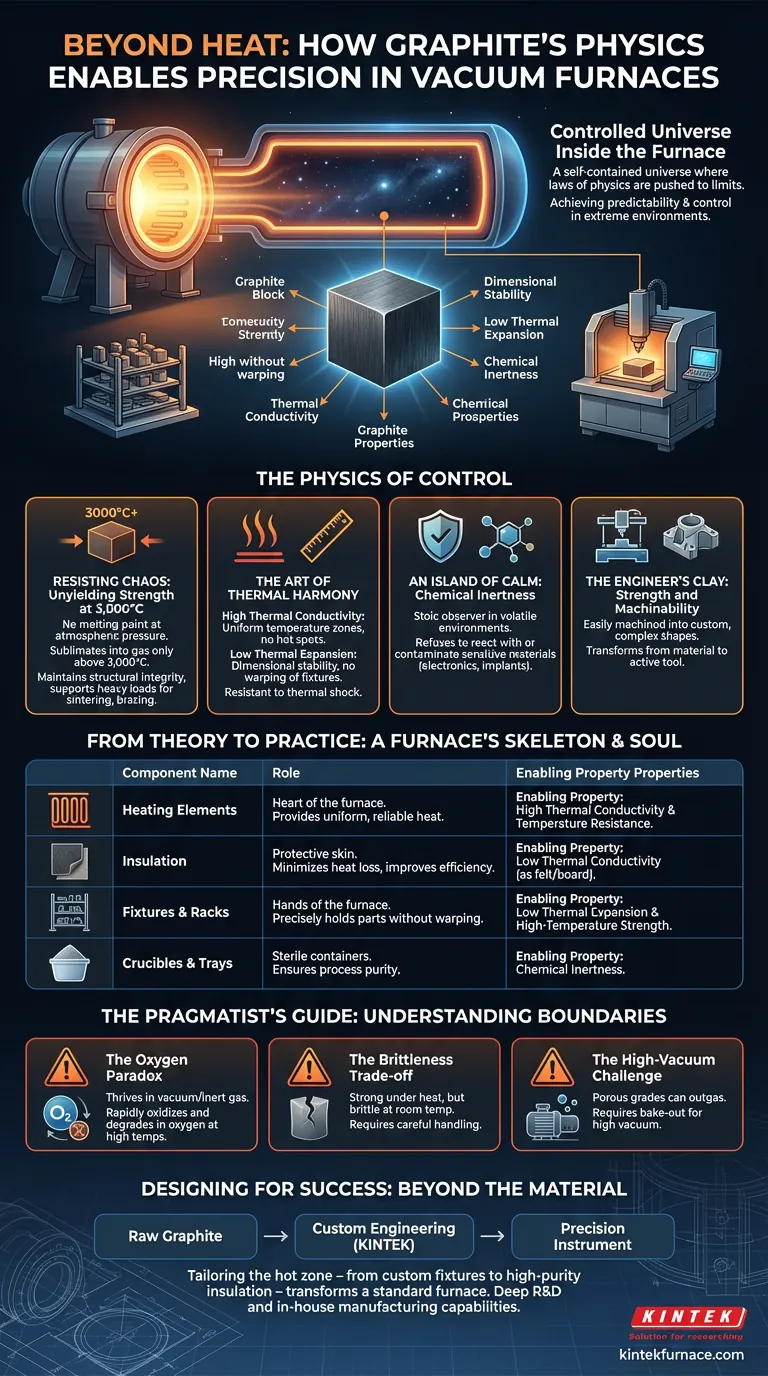
The Controlled Universe Inside the Furnace
A vacuum furnace is more than a hot box. It's a self-contained universe where the laws of physics are pushed to their limits. The engineering challenge is not merely to generate immense heat, but to maintain absolute control in an environment designed to tear lesser materials apart.
At thousands of degrees Celsius, metals warp, soften, and become hyper-reactive. Order dissolves into chaos. The choice of material for the furnace's internal "hot zone" is therefore a psychological one. We seek predictability, stability, and control. We find it in graphite.
The Physics of Control: Why Graphite Tames the Extreme
Graphite is the default choice for vacuum furnace internals because its properties work in concert to create a stable, predictable environment. It doesn't just survive the heat; it provides the structure and control necessary for precision processes.
Resisting Chaos: Unyielding Strength at 3,000°C
Most materials have a breaking point defined by melting. Graphite doesn't. At atmospheric pressure, it bypasses melting entirely, sublimating directly into a gas only above 3,000°C.
This isn't just a high number; it's a guarantee of structural integrity. While refractory metals begin to sag and lose strength, a graphite fixture remains rigid, reliably supporting heavy loads. For processes like sintering, brazing, or annealing, this stability is the bedrock of a repeatable, successful outcome.
The Art of Thermal Harmony
Managing heat is a delicate dance between distribution and stability. Graphite masters both.
- High Thermal Conductivity: Heat flows through graphite easily and evenly. This ensures that heating elements create a uniform temperature zone, eliminating hot spots that could ruin a sensitive component.
- Low Thermal Expansion: While conducting heat superbly, graphite itself barely expands. This dimensional stability is critical. Fixtures, trays, and supports don't warp or shift, meaning the workpiece stays exactly where you placed it.
This combination also gives it immense resistance to thermal shock. It can handle rapid heating and cooling cycles without cracking, enabling faster and more efficient processes.
An Island of Calm: Chemical Inertness
High temperatures are a catalyst for chemical reactions. In this volatile environment, graphite is a stoic observer. It is chemically inert, refusing to react with or contaminate the materials being treated.
For manufacturing sensitive electronics, medical implants, or metallurgical samples, this purity is non-negotiable. Graphite ensures that the only changes to the product are the ones you intended.
The Engineer's Clay: Strength and Machinability
For all its strength and resilience, graphite is surprisingly cooperative. It is relatively easy to machine into complex, custom shapes.
This property is an engineer's dream. It transforms graphite from a passive material into an active tool. An intricate aerospace component needs a perfectly shaped cradle for heat treatment; a unique research sample requires a bespoke container. Graphite can be milled, lathed, and cut to create these elegant, functional solutions.
From Theory to Practice: A Furnace's Skeleton and Soul
These fundamental properties translate directly into the critical components that form a vacuum furnace's hot zone. Each part plays a specific role, made possible by graphite's unique physics.
| Component | Role & Enabling Graphite Property |
|---|---|
| Heating Elements | The heart of the furnace. Provides uniform, reliable heat thanks to graphite's high thermal conductivity and temperature resistance. |
| Insulation | The protective skin (as felt or board). Minimizes heat loss and improves energy efficiency due to its low thermal conductivity in this form. |
| Fixtures & Racks | The hands of the furnace. Precisely holds parts without warping due to its low thermal expansion and high-temperature strength. |
| Crucibles & Trays | The sterile containers. Ensures process purity for powders and small parts thanks to graphite's chemical inertness. |
The Pragmatist's Guide: Understanding Graphite's Boundaries
No material is perfect. Understanding graphite's limitations is key to using it effectively. These aren't weaknesses, but rather rules of engagement.
- The Oxygen Paradox: Graphite thrives in a vacuum or inert gas. Its one true vulnerability is oxygen at high temperatures, which causes it to rapidly oxidize and degrade. The furnace's vacuum is the very environment that unlocks its potential.
- The Brittleness Trade-off: While incredibly strong under heat, solid graphite can be brittle at room temperature. It demands careful handling during loading and unloading to prevent chipping or cracking.
- The High-Vacuum Challenge: Some grades of graphite are porous and can absorb atmospheric gases. In high-vacuum systems, this leads to outgassing, requiring proper bake-out procedures to achieve the necessary vacuum levels.
Designing for Success: Beyond the Material Itself
Simply choosing graphite isn't enough. The grade of the graphite, the design of the component, and the manufacturing precision are what separate a functional hot zone from a high-performance one. A standard fixture may not suffice for a complex research prototype in a CVD system, and a generic heating element may not provide the uniformity needed for advanced materials.
This is where engineering expertise becomes critical. Tailoring the hot zone—from custom-machined fixtures to high-purity insulation—transforms a standard furnace into a precision instrument. Companies like KINTEK, with deep R&D and in-house manufacturing capabilities, provide these custom-engineered furnace solutions, ensuring that every component is perfectly matched to the specific process demands.
Ultimately, graphite is the enabler. It provides the physical foundation of stability and control, allowing scientists and engineers to achieve predictable, repeatable results in the most extreme of thermal environments. The difference between a successful experiment and a costly failure often lies in the details of your furnace's hot zone. For systems engineered to your exact specifications, Contact Our Experts.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
Related Articles
- The Tyranny of Air: How Vacuum Furnaces Forge Perfection by Removing Everything
- The Unseen Advantage: How Vacuum Furnaces Forge Metallurgical Perfection
- Designing for the Void: The Counter-Intuitive Physics of Graphite in Vacuum Furnaces
- Beyond the Heat: The Psychology of Perfect Vacuum Furnace Operation
- The Paradox of Strength: Why Graphite Dominates High-Temperature Vacuum Furnaces




















