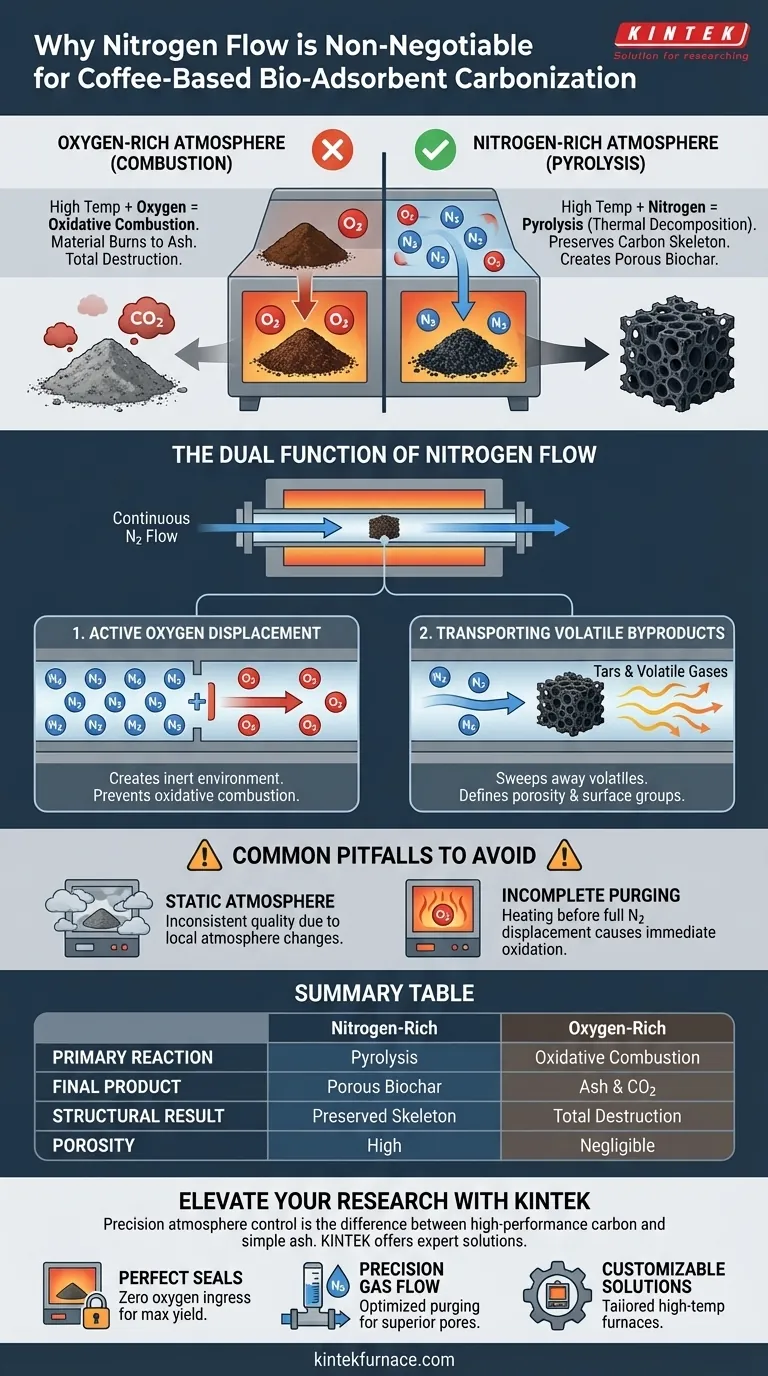
The use of an atmosphere furnace with nitrogen flow is non-negotiable for one specific reason: preventing oxidative combustion. When coffee grounds are heated to high temperatures in the presence of oxygen, they simply burn into ash. The nitrogen flow displaces this oxygen, creating an inert environment that forces the material to undergo pyrolysis—thermal decomposition without burning—thereby preserving the carbon skeleton required for adsorption.
The nitrogen atmosphere acts as a chemical gatekeeper, ensuring that high thermal energy transforms the biomass into a porous, high-carbon structure rather than destroying it through oxidation.
The Critical Role of the Inert Atmosphere
Preventing Material Destruction
The fundamental goal of carbonization is to enrich the carbon content of the coffee grounds. If you attempt this process in a standard oven with air (which contains oxygen), the high temperatures triggers oxidative combustion.
Instead of creating a carbon-rich biochar, the material will react with oxygen to form carbon dioxide and ash. An atmosphere furnace provides the sealed environment necessary to exclude ambient air entirely.
Preserving Structural Integrity
For a bio-adsorbent to be effective, it must maintain a specific physical structure. The inert nitrogen atmosphere protects the structural integrity of the biomass.
This preservation ensures the resulting material retains the mechanical stability needed for filtration or absorption applications.
The Dual Function of Nitrogen Flow
Active Oxygen Displacement
Nitrogen is not merely a passive filler; it is an active displacement agent. A continuous flow creates positive pressure within the furnace tube, pushing out any residual oxygen and preventing outside air from leaking in.
This continuous purging is the only way to guarantee the environment remains strictly inert throughout the entire heating cycle.
Transporting Volatile Byproducts
During pyrolysis, the coffee biomass releases various volatile gases and tars. If these byproducts remain in the chamber, they can re-deposit onto the sample or interfere with the developing pore structure.
The constant nitrogen flow acts as a transport mechanism, sweeping these volatiles away from the sample. This removal is essential for defining the final porosity and surface functional groups of the activated carbon.
Common Pitfalls to Avoid
The Risk of Static Atmosphere
It is a mistake to assume a sealed furnace without flow is sufficient. Without a continuous stream of nitrogen to carry away evolved gases, the local atmosphere around the sample changes, leading to inconsistent quality.
Incomplete Purging
A common error is heating the furnace before the nitrogen has fully displaced the oxygen. The system must be purged thoroughly before the temperature rises to prevent immediate surface oxidation as the reaction begins.
Making the Right Choice for Your Goal
To maximize the efficacy of your coffee-based bio-adsorbents, you must tailor your furnace settings to your specific objectives.
- If your primary focus is Maximizing Carbon Yield: Ensure the furnace seal is perfect and the nitrogen purge is extensive to prevent even trace amounts of oxygen from burning off your material.
- If your primary focus is High Surface Area (Porosity): Maintain a steady, consistent nitrogen flow rate to effectively sweep away tars and volatiles that would otherwise clog the micropores of the biochar.
Precise control of your atmosphere is the difference between creating a high-performance adsorbent and simply burning expensive waste.
Summary Table:
| Factor | Nitrogen-Rich Atmosphere | Oxygen-Rich Atmosphere |
|---|---|---|
| Primary Reaction | Pyrolysis (Thermal Decomposition) | Oxidative Combustion |
| Final Product | Porous Biochar/Activated Carbon | Ash and Carbon Dioxide |
| Structural Result | Preserved Carbon Skeleton | Total Material Destruction |
| Volatile Handling | Effectively Swept Away by Flow | Reactive Interferences |
| Porosity | High (High Surface Area) | Negligible |
Elevate Your Bio-Adsorbent Research with KINTEK
Precision in atmosphere control is the difference between high-performance carbon and simple ash. At KINTEK, we understand the critical nature of inert environments for pyrolysis and activation. Backed by expert R&D and manufacturing, we provide high-performance Atmosphere Furnaces, Tube Furnaces, and Vacuum Systems specifically designed for researchers and industrial manufacturers.
Our systems offer:
- Perfect Seals: Ensuring zero oxygen ingress for maximum carbon yield.
- Precision Gas Flow: Optimized nitrogen purging to develop superior pore structures.
- Customizable Solutions: Tailored high-temp furnaces to meet your unique biomass processing needs.
Ready to optimize your carbonization process? Contact us today to find the perfect furnace solution!
Visual Guide
References
- A Coffee-Based Bioadsorbent for CO2 Capture from Flue Gas Using VSA: TG-Vacuum Tests. DOI: 10.3390/en18153965
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the primary purposes of a controlled atmosphere furnace? Achieve Precise Material Processing and Protection
- How does an atmosphere box furnace contribute to the synthesis and preparation of new energy materials? Unlock Precision for Advanced Energy Solutions
- What is the function of a high-pressure Argon atmosphere? Master Complex Alloy Purity with Precision Melting
- What materials are used in the construction of a retort furnace? Discover Key Components for High-Temp Control
- What is the function of a high-temperature pyrolysis furnace in the preparation of magnetic Fe3O4/biochar nanoparticles?
- Why is a high-temperature atmosphere annealing furnace used after depositing Cu-doped In2O3 thin films?
- What role do atmosphere furnaces play in metal processing? Prevent Oxidation and Enhance Surface Properties
- Why is an argon atmosphere used? Ensure Material Purity in High-Temp Processes



















