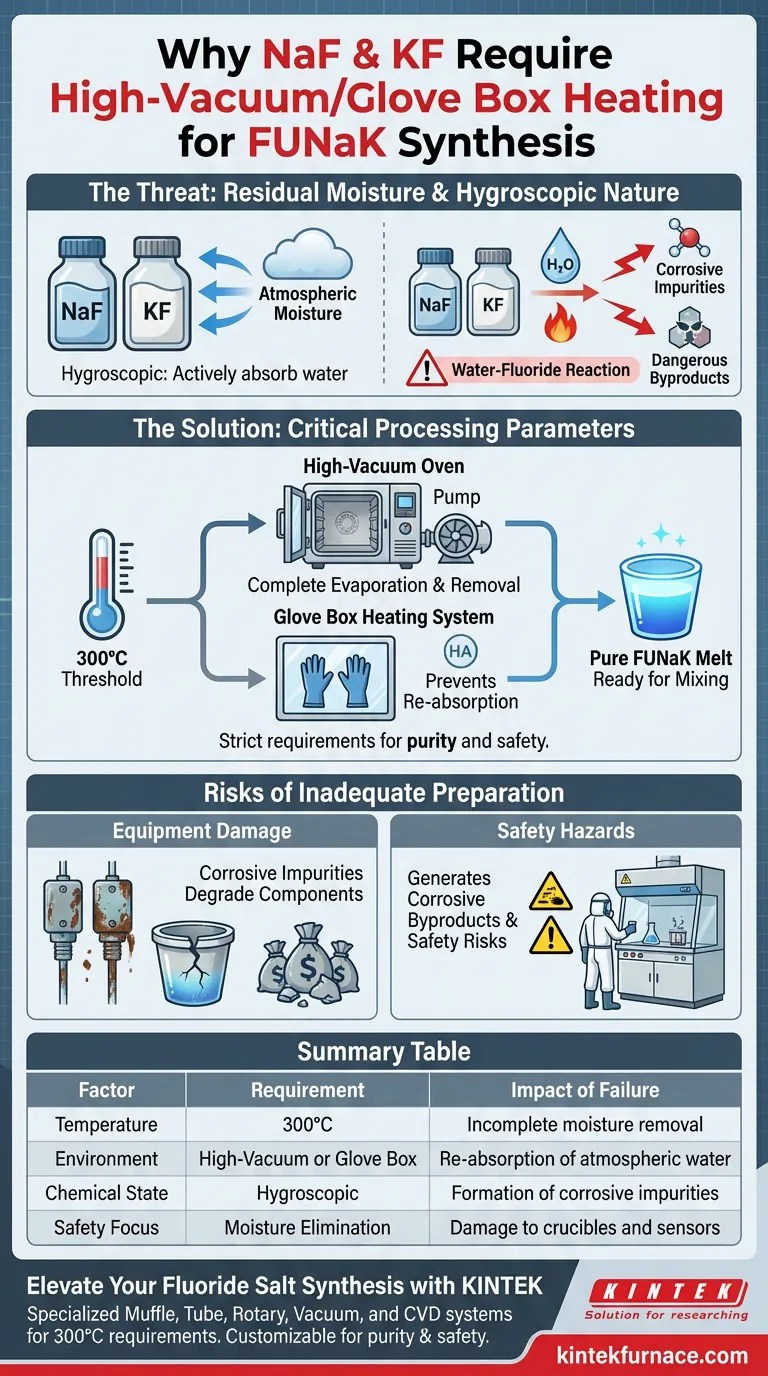
Sodium Fluoride (NaF) and Potassium Fluoride (KF) require processing in a high-vacuum or glove box heating system because they are inherently hygroscopic. Before mixing, these raw materials must be heated to 300°C to force the complete evaporation of residual moisture. This step is non-negotiable for preventing chemical reactions between water and fluoride salts that create dangerous impurities.
The strict requirement for vacuum heating is not just about purity; it is a critical safety measure. By eliminating moisture, you prevent the formation of corrosive byproducts that degrade the FLiNaK melt and damage sensitive laboratory equipment.

The Threat of Residual Moisture
The primary challenge in synthesizing FUNaK is the chemical stability of the raw ingredients in the presence of water.
The Hygroscopic Nature of Raw Materials
Both NaF and KF are hygroscopic, meaning they actively absorb moisture from the surrounding atmosphere. Even if the salts appear physically dry, they likely contain significant amounts of absorbed water at a molecular level.
The Water-Fluoride Reaction
If moisture is present during the melting process, water reacts chemically with the fluoride salts. This reaction generates corrosive impurities rather than a pure fluoride melt.
Compromising Melt Purity
These impurities fundamentally alter the chemistry of the melt. If the raw materials are not effectively dried, the final product will not meet the purity standards required for accurate experimental data or application.
Critical Processing Parameters
To ensure the integrity of the synthesis, specific environmental conditions must be met prior to mixing.
The 300°C Threshold
Standard drying is insufficient; the materials must be heated to 300°C. This high temperature is required to ensure the complete evaporation of all residual moisture trapped within the salt structure.
The Necessity of Vacuum or Glove Box Systems
Heating must occur within a vacuum oven or a glove box heating system. This controlled environment facilitates the removal of evolved water vapor and prevents the salts from re-absorbing moisture from the air during the heating process.
Risks of Inadequate Preparation
Skipping or rushing the pre-mixing treatment involves significant trade-offs that jeopardize both the experiment and the lab environment.
Equipment Damage
The corrosive impurities formed by the reaction of water and fluoride salts are highly aggressive. They can degrade crucibles, sensors, and the internal components of the heating rig, leading to costly equipment failure.
Safety Hazards
The generation of corrosive byproducts poses a safety risk to laboratory personnel. Ensuring the raw materials are moisture-free is a primary control measure for maintaining a safe operating environment.
Ensuring Successful Synthesis
To achieve a high-quality FUNaK melt, you must strictly adhere to the drying protocol.
- If your primary focus is Melt Purity: Ensure the heating cycle reaches a full 300°C to eliminate all potential reactants that could contaminate the final stoichiometry.
- If your primary focus is Equipment Safety: Utilize a high-vacuum or glove box system to prevent the formation of corrosive agents that attack containment vessels and heating elements.
Strict moisture control is the foundation of both experimental success and laboratory safety in fluoride salt synthesis.
Summary Table:
| Factor | Requirement | Impact of Failure |
|---|---|---|
| Temperature | 300°C | Incomplete moisture removal |
| Environment | High-Vacuum or Glove Box | Re-absorption of atmospheric water |
| Chemical State | Hygroscopic | Formation of corrosive impurities |
| Safety Focus | Moisture Elimination | Damage to crucibles and sensors |
Elevate Your Fluoride Salt Synthesis with KINTEK
Don't compromise your melt purity or lab safety with inadequate heating systems. Backed by expert R&D and precision manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous 300°C vacuum requirements for NaF and KF processing. Our lab high-temp furnaces are fully customizable to ensure your FUNaK synthesis is free from corrosive impurities and equipment-damaging moisture.
Ready to optimize your high-temperature research? Contact our specialists today to find the perfect thermal solution for your unique needs!
Visual Guide
References
- Maxime Fache, O. Beneš. Thermophysical Properties of FUNaK (NaF-KF-UF4) Eutectics. DOI: 10.3390/ma17112776
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Magnesium Extraction and Purification Condensing Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What benefits does vacuum carburizing offer for parts with complex shapes? Minimize Distortion and Boost Performance
- Why is a laboratory vacuum drying oven necessary for sodium-ion battery half-cells? Achieve Peak Battery Performance
- What are the process advantages of cyclic vacuum annealing and oxidation? Maximize Carbon Chain Yield up to 48%
- What are the primary process objectives of using a vacuum annealing furnace for treating HEA multilayer films?
- What are the key benefits of using a vacuum sintering furnace? Achieve Superior Material Purity and Process Control
- What are the primary applications of a vacuum heat treatment furnace? Achieve Superior Metallurgical Outcomes
- What are the advantages of a mesh belt brazing furnace vs vacuum? Optimize High-Volume Stainless Steel Production
- Why is dual monitoring used for Tantalum annealing? Achieve 20K Precision in Vacuum Furnaces



















