High temperature control stability is the prerequisite for valid kinetic calculations. When using the Kissinger-Akahira-Sunose (KAS) method, the accuracy of your results depends entirely on observing the precise displacement of characteristic temperature points across varying heating rates, such as 10, 20, and 30°C/min. Without strict stability, the oxidation reaction stages of your sample (specifically coal) become irreproducible, leading to calculation deviations caused by thermal hysteresis.
The KAS method calculates activation energy by comparing temperature shifts across different heating rates. High stability is required to minimize thermal hysteresis, ensuring that data variations are caused by the reaction kinetics itself, not by instrumental error or lag.
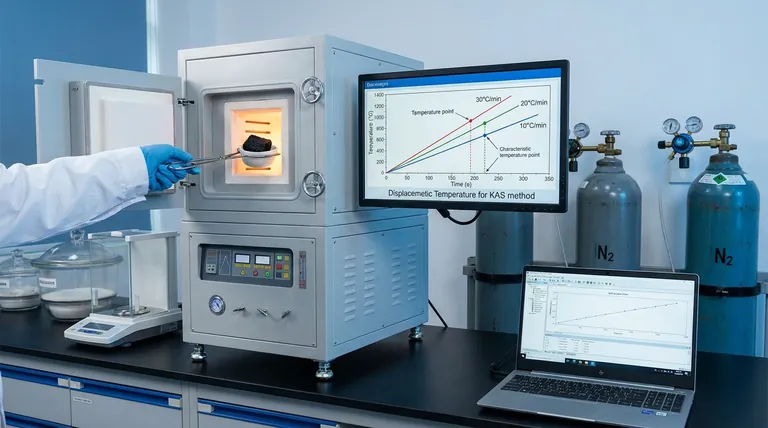
The Mechanics of the KAS Method
Dependence on Heating Rate Variation
The KAS method does not rely on a single measurement; it relies on the comparative analysis of data collected at different speeds.
Standard protocols often utilize heating rates of 10, 20, and 30°C/min to induce shifts in the sample's reaction profile.
Observing Temperature Displacement
The core mathematical principle involves tracking the displacement of characteristic temperature points as the heating rate changes.
If the instrument cannot maintain a linear and precise temperature ramp, the specific points required for the calculation become unreliable.
Why Stability Dictates Accuracy
Ensuring Reproducibility of Reaction Stages
For the KAS equation to be valid, the oxidation reaction stages of the coal sample must be reproducible across all tested heating rates.
If the equipment fluctuates, the reaction environment changes between runs, making it impossible to correlate the data from the 10°C/min run with the 30°C/min run scientifically.
Minimizing Thermal Hysteresis
The primary threat to accuracy in this context is thermal hysteresis, which is a lag between the input temperature and the sample's actual temperature.
High control stability minimizes this hysteresis, ensuring that the recorded temperature accurately reflects the thermal state of the sample during oxidation.
Reducing Calculation Deviations
Any instability in the temperature control introduces calculation deviations that propagate through the KAS equation.
These deviations distort the final activation energy values, rendering the resulting kinetic parameters scientifically inaccurate.
Common Pitfalls to Avoid
The Illusion of Linear Heating
A common mistake is assuming that setting a heating rate guarantees that rate is achieved without fluctuation.
In lower-quality equipment, the actual heating profile may oscillate, introducing noise that the KAS method interprets as kinetic data, leading to false activation energy values.
Neglecting Thermal Lag
Failing to account for the equipment's control stability can lead to data that appears smooth but suffers from significant thermal lag.
This lag artificially shifts the characteristic temperature points, causing the KAS method to miscalculate the reaction kinetics.
Making the Right Choice for Your Experiment
To ensure the scientific validity of your coal oxidation kinetic parameters, evaluate your equipment based on your specific goals:
- If your primary focus is reproducible data: Ensure your equipment has high temperature control stability to maintain consistent reaction stages across different heating rates.
- If your primary focus is calculation accuracy: Prioritize equipment that explicitly minimizes thermal hysteresis to prevent deviations in your KAS calculations.
Precision in temperature control is not just an equipment feature; it is the foundation of accurate kinetic modeling.
Summary Table:
| Factor | Impact on KAS Method | Requirement for Accuracy |
|---|---|---|
| Heating Rate | Drives displacement of temperature points | Precise linear ramps (e.g., 10, 20, 30°C/min) |
| Thermal Hysteresis | Causes lag between input and actual sample temp | Must be minimized via high control stability |
| Reproducibility | Ensures reaction stages are consistent across runs | Uniform environment for all heating rate trials |
| Data Integrity | Prevents calculation deviations in activation energy | High stability to eliminate instrumental noise |
Elevate Your Kinetic Research with KINTEK Precision
Don’t let thermal hysteresis compromise your activation energy calculations. KINTEK provides industry-leading thermal solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, engineered for the extreme temperature control stability required by the Kissinger-Akahira-Sunose (KAS) method.
Our customizable high-temperature lab furnaces are backed by expert R&D and precision manufacturing to ensure your reaction stages are perfectly reproducible and your data is scientifically sound.
Ready to eliminate calculation deviations?
Contact KINTEK Experts Today to find the perfect furnace for your unique laboratory needs.
References
- Baoshan Jia, Xian Wu. Effects of pre-oxidation temperature and air volume on oxidation thermogravimetric and functional group change of lignite. DOI: 10.1371/journal.pone.0316705
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the function of a flash furnace in sludge treatment? Essential Thermal Preparation for Phosphorus Recovery
- Why is 600 °C critical for ZIF-8 carbonization? Achieve Optimal Surface Area and Functional Group Retention
- What are the specific equipment operational requirements for the SRS process? Unlock Precise Strain Engineering
- Why are continuous furnaces ideal for high-volume manufacturing? Boost Throughput and Consistency
- What are the advantages of mastering the sintering step? Achieve Cost Savings and Complex Designs
- Why are high-precision constant temperature baths necessary? Unlock Accurate Fiber Optic Sensor Calibration
- What is the significance of preheating UHPC molds? Ensure Safety & Longevity with High-Temp Furnaces
- What is the primary function of a vacuum drying oven? Key to Composite Anode Slurry Preparation














