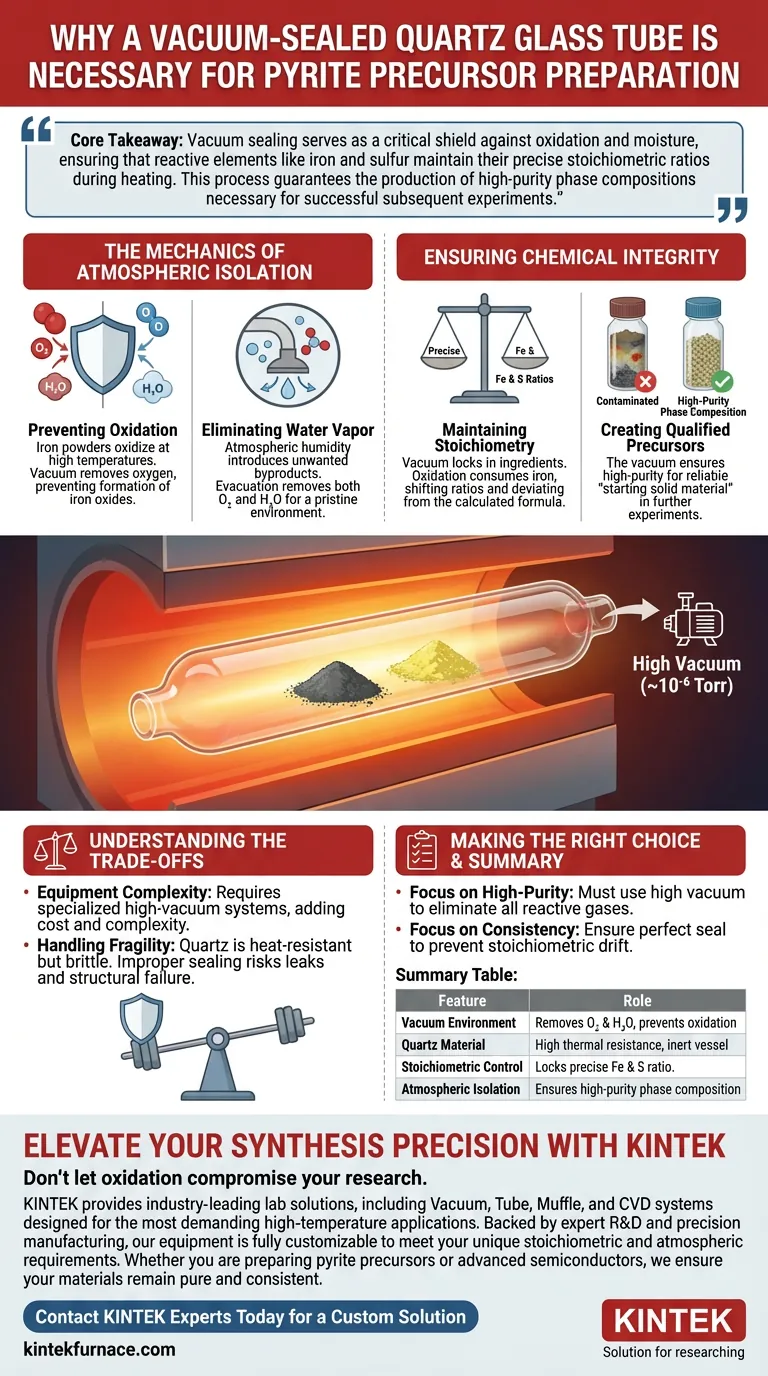
The use of a vacuum-sealed quartz glass tube is a fundamental requirement for maintaining chemical purity. This method creates an isolated environment that completely separates iron and sulfur powders from the surrounding atmosphere. Without this barrier, the high temperatures required for synthesis would trigger immediate oxidation, irreversibly altering the chemical composition of your materials.
Core Takeaway Vacuum sealing serves as a critical shield against oxidation and moisture, ensuring that reactive elements like iron and sulfur maintain their precise stoichiometric ratios during heating. This process guarantees the production of high-purity phase compositions necessary for successful subsequent experiments.
The Mechanics of Atmospheric Isolation
Preventing Oxidation
When synthesizing precursors such as troilite (FeS), you are typically working with iron and sulfur powders. Iron is highly susceptible to oxidation when exposed to high temperatures.
If air is present within the reaction vessel, oxygen will react with the iron to form iron oxides rather than the desired iron sulfide. A vacuum-sealed tube removes this oxygen source entirely.
Eliminating Water Vapor
Beyond simple oxygen, atmospheric air contains humidity. Water vapor can introduce hydrogen and oxygen into the reaction, leading to unwanted byproducts.
Evacuating the tube to a high vacuum level (approximately $10^{-6}$ Torr) ensures the complete removal of both oxygen and water vapor. This creates a pristine environment where only the intended reactants interact.
Ensuring Chemical Integrity
Maintaining Stoichiometry
Solid-state synthesis relies on precise ratios of ingredients, known as stoichiometry.
If oxidation occurs, a portion of your iron is consumed by oxygen rather than sulfur. This shifts the ratio of the remaining elements, resulting in a product that deviates from your calculated formula. Vacuum sealing locks the ratio in place.
Creating Qualified Precursors
The goal of this process is often to create a "starting solid material" for further complex procedures, such as hydrothermal experiments.
If the initial precursor is contaminated with oxides or has an incorrect phase composition, all subsequent experimental steps will be compromised. The vacuum tube ensures the high-purity phase composition required to make the precursor a reliable building block.
Understanding the Trade-offs
Equipment Complexity
Achieving the necessary vacuum level requires specialized high-vacuum systems. This adds a layer of complexity and cost compared to open-air synthesis methods.
Handling Fragility
Quartz glass is robust against heat but brittle against physical stress. Sealing these tubes requires skill; an improper seal can lead to leaks (reintroducing oxygen) or structural failure under pressure.
Making the Right Choice for Your Goal
To ensure your synthesis yields useful data, apply these principles based on your specific objectives:
- If your primary focus is high-purity phase composition: You must utilize a high vacuum system to evacuate the tube to at least $10^{-6}$ Torr to eliminate all traces of reactive gases.
- If your primary focus is material consistency: Ensure the quartz tube is perfectly sealed to prevent stoichiometric drift caused by the loss of volatile sulfur or the intrusion of oxygen.
By strictly controlling the atmosphere, you transform volatile raw powders into stable, high-quality experimental foundations.
Summary Table:
| Feature | Role in Pyrite Precursor Preparation |
|---|---|
| Vacuum Environment | Removes oxygen and humidity to prevent unwanted oxidation of iron powders. |
| Quartz Material | Provides high thermal resistance while maintaining an inert reaction vessel. |
| Stoichiometric Control | Prevents chemical drift by locking the precise ratio of iron and sulfur. |
| Atmospheric Isolation | Eliminates reactive gas intrusion to ensure high-purity phase composition. |
Elevate Your Synthesis Precision with KINTEK
Don’t let oxidation compromise your research. KINTEK provides industry-leading lab solutions, including Vacuum, Tube, Muffle, and CVD systems designed for the most demanding high-temperature applications.
Backed by expert R&D and precision manufacturing, our equipment is fully customizable to meet your unique stoichiometric and atmospheric requirements. Whether you are preparing pyrite precursors or advanced semiconductors, we ensure your materials remain pure and consistent.
Contact KINTEK Experts Today for a Custom Solution
Visual Guide
References
- Е. V. Коvalchuk, I. V. Vikentyev. Gold and Arsenic in Pyrite and Marcasite: Hydrothermal Experiment and Implications to Natural Ore-Stage Sulfides. DOI: 10.3390/min14020170
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the typical working temperature ranges for lab tube furnaces? Find the Right Furnace for Your Process
- What core process conditions does a tube furnace provide during Ni-TiN catalyst calcination? Master Precise Catalysis
- How does a vertical tube furnace achieve energy efficiency? Key Design Features for Lower Energy Costs
- What critical conditions does a high-precision tube furnace provide? Optimize Catalyst Reduction & Particle Control
- What maintenance practices are recommended for a multi zone tube furnace? Ensure Safety and Precision in Your Lab
- What role does a laboratory tube furnace system play in the catalytic pyrolysis of LLDPE? Enhancing Yield and Precision
- What role does a high-temperature tube furnace play in N-CP synthesis? Mastering Precision Carbonization
- What are the different types of tubular furnaces? Choose the Right One for Your Lab



















