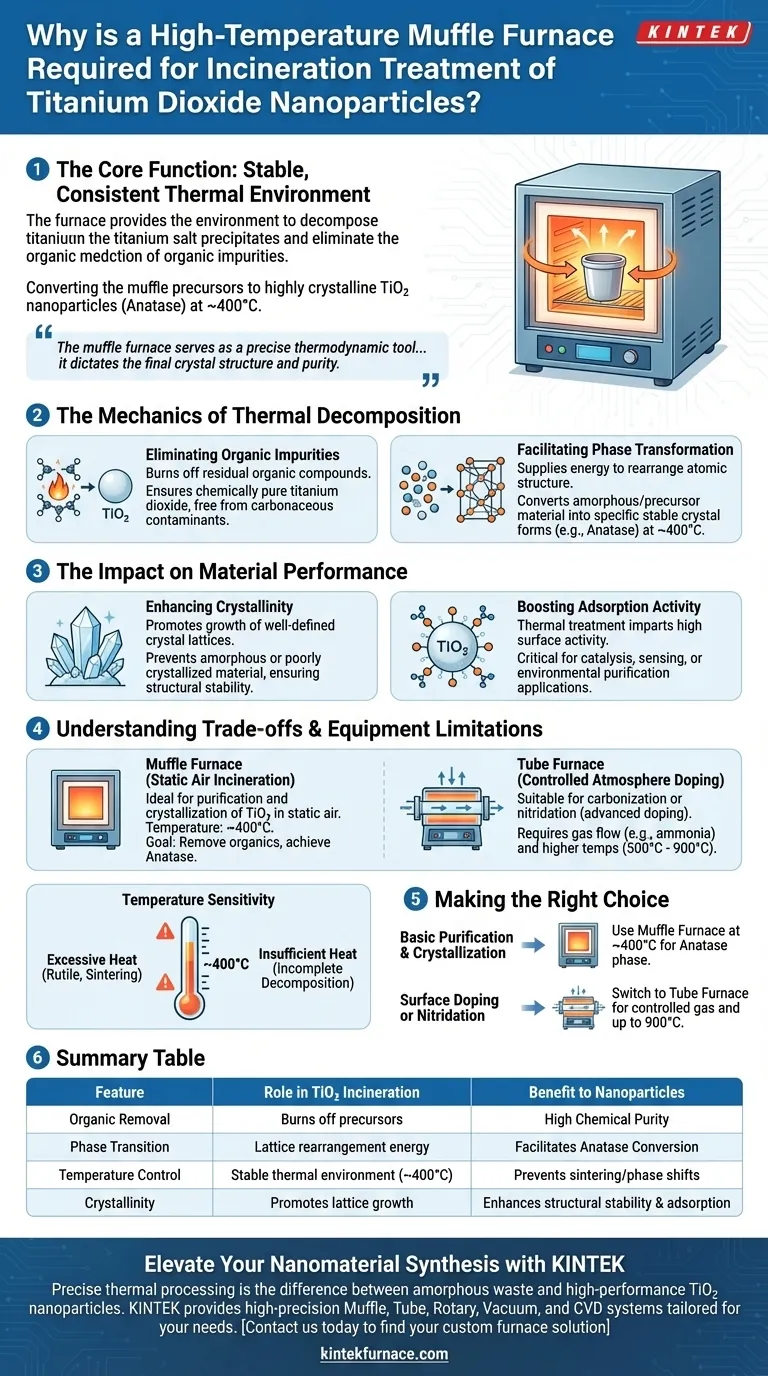
A high-temperature muffle furnace is required to provide the stable, consistent thermal environment necessary to decompose titanium salt precipitates and effectively eliminate organic impurities. This process, typically conducted at approximately 400°C, drives the phase transformation that converts raw precursors into highly crystalline titanium dioxide (TiO2) nanoparticles, often in the anatase form.
The muffle furnace serves as a precise thermodynamic tool that does more than simply dry the material; it dictates the final crystal structure and purity required for the nanoparticles to exhibit high adsorption activity and structural stability.

The Mechanics of Thermal Decomposition
Eliminating Organic Impurities
The primary function of the incineration treatment is purification. As the furnace heats the titanium salt precipitates, it burns off residual organic compounds left over from the synthesis process.
This ensures the final product is chemically pure titanium dioxide, free from carbonaceous contaminants that could hinder performance.
Facilitating Phase Transformation
Raw titanium precipitates do not naturally possess the crystal structure required for high-performance applications. The steady heat of the muffle furnace supplies the energy needed to rearrange the atomic structure.
At temperatures around 400°C, this process converts the amorphous or precursor material into specific, stable crystal forms, most notably anatase.
The Impact on Material Performance
Enhancing Crystallinity
High crystallinity is directly linked to the stability and effectiveness of the nanoparticle. The thermal treatment promotes the growth of well-defined crystal lattices.
Without this high-temperature step, the material would likely remain amorphous or poorly crystallized, significantly degrading its physical properties.
Boosting Adsorption Activity
The specific crystal forms generated during incineration are not just structural; they are functional. The primary reference highlights that this thermal treatment imparts high adsorption activity to the material.
This surface activity is critical if the nanoparticles are intended for use in catalysis, sensing, or environmental purification applications.
Understanding the Trade-offs and Equipment Limitations
Muffle Furnace vs. Tube Furnace
It is vital to distinguish between incineration (oxidation) and doping (modification). Muffle furnaces are ideal for static air incineration to remove organics and crystallize TiO2.
However, if your goal involves carbonization or nitridation (as seen in advanced doping processes), a muffle furnace is generally unsuitable. These processes typically require a tube furnace to manage gas flow (such as ammonia) and precise gradients between 500°C and 900°C.
Temperature Sensitivity
While 400°C is a standard baseline for obtaining anatase, deviating from this temperature changes the outcome.
Excessive heat can trigger unwanted phase transitions (e.g., to rutile) or induce sintering, which reduces surface area. Insufficient heat will fail to fully decompose the precursors or remove all impurities.
Making the Right Choice for Your Goal
To ensure you are applying the correct thermal treatment for your specific nanomaterial requirements, consider the following:
- If your primary focus is basic purification and crystallization: Utilize a muffle furnace at ~400°C to remove organics and achieve the anatase crystal phase.
- If your primary focus is surface doping or nitridation: Switch to a tube furnace to allow for controlled gas atmospheres and higher temperature ranges (up to 900°C).
By matching the furnace type and temperature profile to your specific chemical targets, you ensure the production of nanoparticles with optimized structural and functional integrity.
Summary Table:
| Feature | Role in TiO2 Incineration | Benefit to Nanoparticles |
|---|---|---|
| Organic Removal | Burns off carbonaceous precursors | Ensures high chemical purity |
| Phase Transition | Provides energy for lattice rearrangement | Facilitates conversion to Anatase form |
| Temperature Control | Maintains stable thermal environment (~400°C) | Prevents sintering & unwanted phase shifts |
| Crystallinity | Promotes growth of crystal lattices | Enhances structural stability & adsorption |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise thermal processing is the difference between amorphous waste and high-performance TiO2 nanoparticles. Backed by expert R&D and world-class manufacturing, KINTEK provides high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for your laboratory’s specific incineration or doping needs.
Whether you require static air oxidation or controlled-atmosphere nitridation, our customizable high-temperature furnaces ensure the structural and functional integrity of your materials. Contact us today to find your custom furnace solution.
Visual Guide
References
- Duaa Ayad Yass, Ahmed Mohammed Abbas. ADSORPTION OF CONGO RED DYE ON ACTIVATED GRAPHITE AND ITS COMPOSITE, AN ISOTHERMAL AND THERMODYNAMIC STUDY. DOI: 10.32737/0005-2531-2025-2-70-78
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What function does high-temperature calcination in a muffle furnace serve for TiO2? Expert Phase Control Guide
- What is the function of a box muffle furnace in nanoparticle stabilization? Optimize Active Ingredient Efficacy
- Why is a high-precision muffle furnace required for BCZT xerogel pre-calcination? Ensure Pure Phase and Reactivity
- What temperature considerations are important for muffle furnaces? Optimize Performance and Longevity
- Why is a double-chamber device preferred over a standard electric furnace for sintering? Achieve Oxidation-Free Results
- What is the significance of using a box resistance furnace for the 900 °C sintering of high-entropy alloys?
- What factors should be considered when purchasing a box type electric furnace? Ensure Optimal Performance for Your Lab
- What core role does a high-temperature box resistance furnace play in the production of doped Nickel Oxide nanopowders?



















