In the context of pharmaceutical analysis, a muffle furnace is essential because it provides the highly controlled, high-temperature environment required to reliably quantify a material's composition. These tests for moisture, ash, and volatile content are not just procedural; they are fundamental indicators of a drug's purity, stability, and adherence to strict regulatory standards.
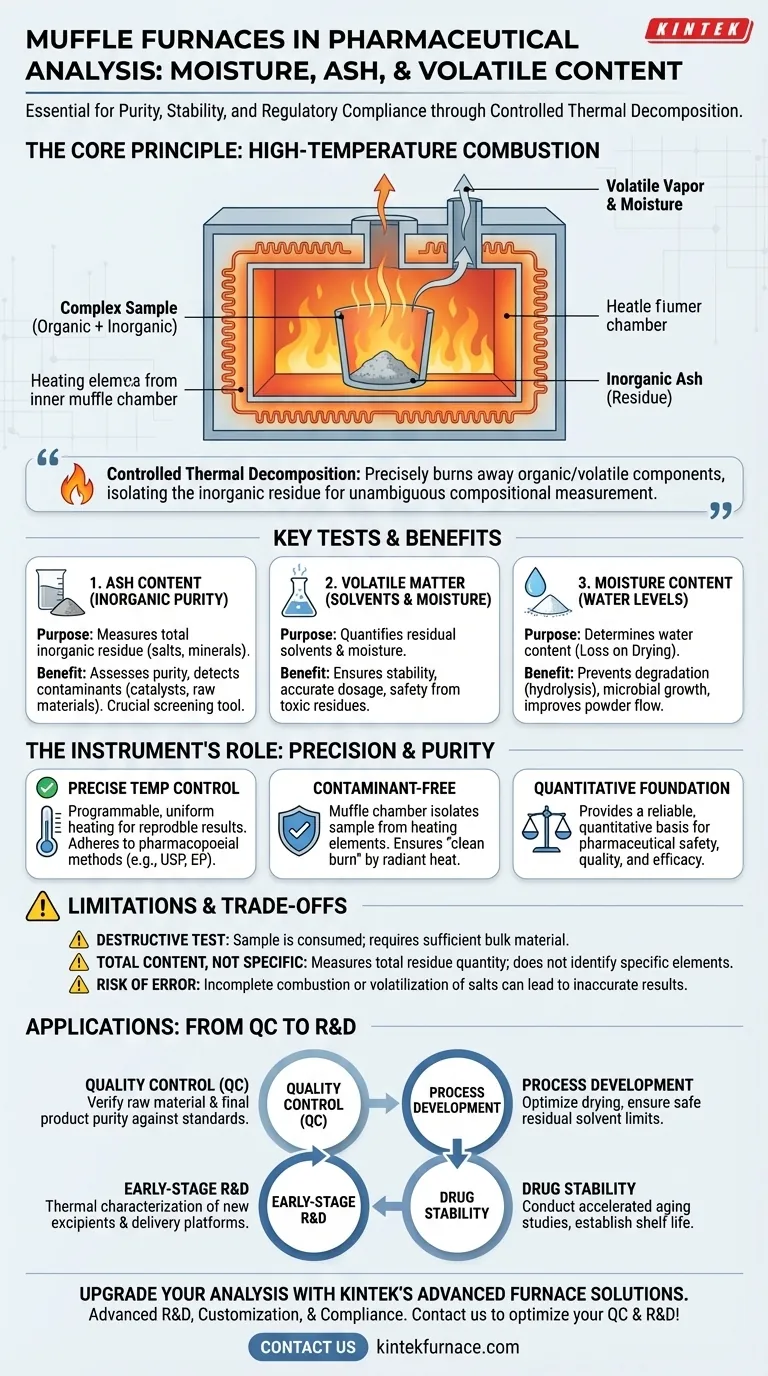
The core value of a muffle furnace in pharmaceuticals lies in its ability to perform controlled thermal decomposition. By precisely burning away all organic and volatile components, it isolates the non-combustible inorganic residue (ash), providing an unambiguous, quantitative measure of a material's fundamental composition and purity.

The Furnace as an Analytical Instrument
A muffle furnace is far more than a simple oven. Its design and operation are geared toward providing reproducible and contaminant-free results, which is non-negotiable in a regulated industry.
The Principle of High-Temperature Combustion
The primary function is to heat a sample to a specific, high temperature until all organic matter and volatile compounds are either destroyed through combustion or driven off as vapor.
This process, known as ignition or ashing, is destructive by design. It simplifies a complex organic-inorganic mixture into a single, measurable component: the inorganic residue.
The Critical Role of Temperature Control
Pharmaceutical analysis demands absolute consistency. Muffle furnaces offer programmable and highly uniform heating, ensuring that the conditions of a test can be replicated perfectly.
This precision is critical for adhering to validated methods outlined in official pharmacopoeias (e.g., USP, EP), which specify exact temperatures and durations for tests like "Residue on Ignition."
Isolating the Analytical Target
The term "muffle" refers to the furnace's inner chamber, which isolates the sample from direct contact with the heating elements.
This prevents contamination from the elements themselves and ensures that the sample is transformed only by radiant heat, providing a "clean burn" that is essential for accurate analytical results.
Deconstructing Key Pharmaceutical Tests
The data from a muffle furnace directly impacts drug quality, safety, and efficacy. Each test reveals a different piece of the compositional puzzle.
Determining Ash Content (Inorganic Purity)
Ash is the inorganic residue—such as salts, minerals, or metallic impurities—that remains after complete combustion of the sample.
This measurement is a direct proxy for inorganic purity. High ash content can signal contamination from manufacturing catalysts, processing aids, or impurities from raw materials. For most purified organic drug substances, the expected ash content is nearly zero.
Measuring Volatile Matter
Volatile matter includes not only moisture but also residual solvents left over from the synthesis or purification process.
Excessive volatile content is a major concern. It can affect the stability of the drug, impact the accuracy of the dosage by contributing extra weight, and pose safety risks if the residual solvents are toxic.
Quantifying Moisture Content
While other methods exist, a furnace can be used for "Loss on Drying" tests to determine water content. Water is a key factor in chemical stability, as it can promote hydrolysis and degradation.
Moisture also affects the physical properties of powders, such as their ability to flow during tablet manufacturing, and can create an environment for microbial growth.
Understanding the Trade-offs and Limitations
While powerful, the technique has inherent characteristics that are important to understand for proper application.
It Is a Destructive Test
The sample used in an ashing test is completely destroyed. This means the method is only suitable for bulk characterization where sufficient material is available.
It Measures Total, Not Specific, Content
Ash analysis tells you the total quantity of inorganic residue, but it does not identify the specific elements or compounds present.
It serves as a crucial screening tool. If the ash content is above the specified limit, further investigation using techniques like atomic spectroscopy (AAS, ICP-MS) is required to identify the contaminants.
Risk of Incomplete Combustion or Loss
If the temperature is too low or the time is too short, organic material may not fully combust, leading to an inaccurately high ash value.
Conversely, some inorganic salts can decompose or volatilize at extremely high temperatures, which could lead to an underestimation of the true ash content if the method is not carefully validated.
How to Apply This to Your Quality & Research Goals
Using a muffle furnace effectively depends entirely on your specific analytical objective.
- If your primary focus is routine quality control: Use muffle furnace ash testing as a non-negotiable method for verifying raw material purity and final product consistency against pharmacopoeial standards.
- If your primary focus is process development: Use volatile content analysis to optimize drying steps and ensure residual solvents are well within safe, specified limits.
- If your primary focus is drug stability: Employ the furnace to conduct accelerated aging studies that reveal potential degradation pathways and help establish a product's shelf life.
- If your primary focus is early-stage R&D: Leverage the furnace for the thermal characterization of new excipients, drug substances, and innovative drug delivery platforms like sintered implants.
Ultimately, mastering the use of a muffle furnace provides an undeniable, quantitative foundation for ensuring pharmaceutical safety, quality, and efficacy.
Summary Table:
| Test Type | Purpose | Key Benefit |
|---|---|---|
| Ash Content | Measures inorganic residue | Assesses purity and detects contaminants |
| Volatile Matter | Quantifies solvents and moisture | Ensures stability and dosage accuracy |
| Moisture Content | Determines water levels | Prevents degradation and microbial growth |
Upgrade your pharmaceutical analysis with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems tailored for precise thermal decomposition and compliance with pharmacopoeial standards. Our deep customization capabilities ensure your unique experimental needs are met, enhancing drug purity, stability, and safety. Contact us today to discuss how our furnaces can optimize your quality control and R&D processes!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How does a muffle furnace contribute to kaolin-modified biochar? Optimize Pyrolysis & Mineral Integration
- What is the primary use of a muffle furnace in the assembly of side-heated resistive gas sensors? Expert Annealing Guide
- Why is a muffle furnace used to determine the ash content of biochar? Master Your Material Purity Analysis
- How does a stainless steel reactor function within a muffle furnace for PET to graphene? Master Carbon Synthesis
- Why are precision stirring and drying equipment necessary for photocatalytic materials? Master Microstructure Control



















