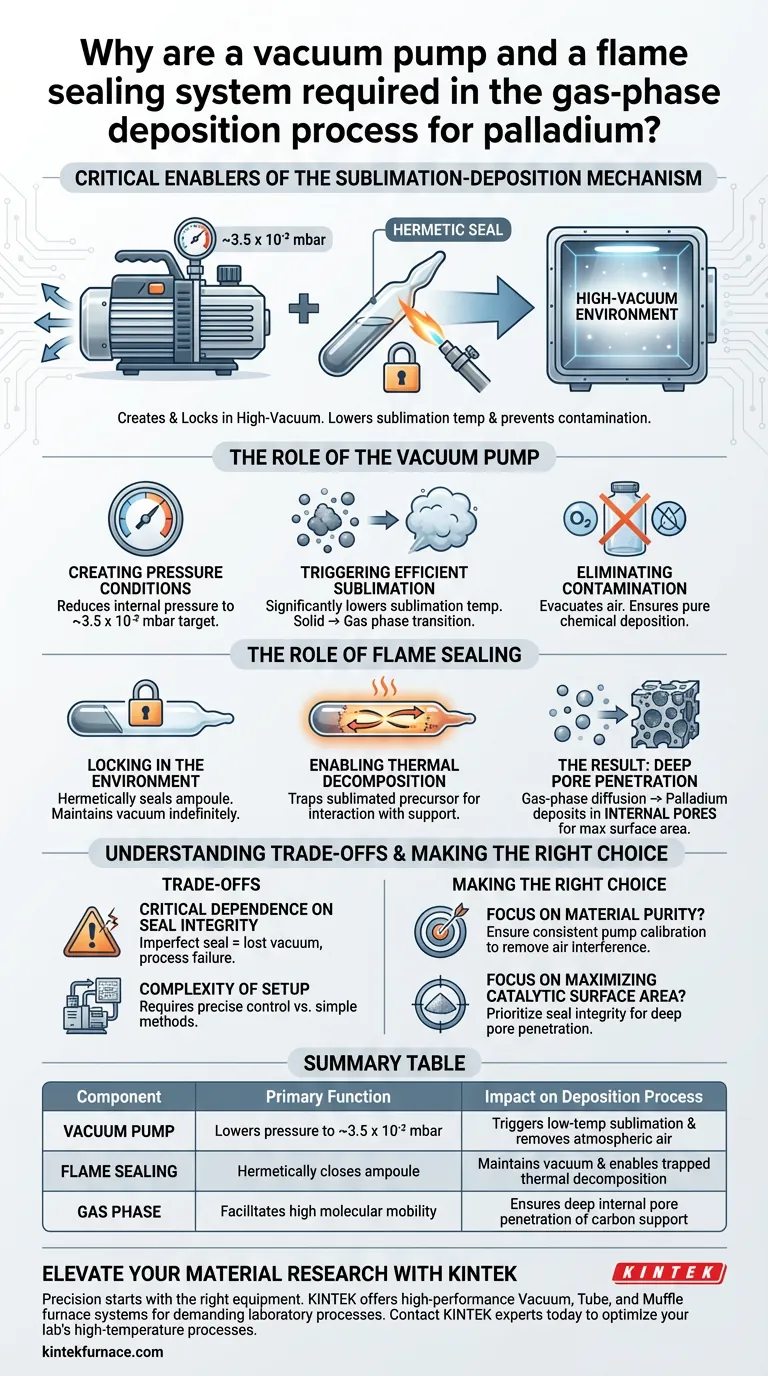
The vacuum pump and flame sealing system are the critical enablers of the sublimation-deposition mechanism. Together, they create and permanently lock in a high-vacuum environment (approximately 3.5 x 10^-2 mbar) inside the reaction ampoule. This specific environment is required to lower the sublimation temperature of the palladium precursor and prevent atmospheric contamination during the deposition process.
By manipulating pressure and isolating the system, these tools allow the palladium precursor to bypass the liquid phase and diffuse as a gas, ensuring it penetrates the deep internal pores of the carbon support.

The Role of the Vacuum Pump
Creating the Necessary Pressure Conditions
The primary function of the vacuum pump is to reduce the internal pressure of the ampoule containing the precursor and carbon support. It targets a specific low-pressure environment of roughly 3.5 x 10^-2 mbar.
Triggering Efficient Sublimation
Reducing the pressure fundamentally alters the physical behavior of the palladium precursor. The vacuum significantly lowers the sublimation temperature, allowing the solid precursor to transition directly into a gas phase without requiring excessive heat.
Eliminating Contamination
The pump evacuates air from the ampoule prior to the reaction. This removal of atmospheric gases eliminates air interference, ensuring that the chemical deposition is pure and undisturbed by oxygen or moisture.
The Role of Flame Sealing
Locking in the Environment
Once the vacuum pump achieves the target pressure, the flame sealing system hermetically seals the ampoule. This converts the open container into a closed, isolated system that maintains the vacuum indefinitely.
Enabling Thermal Decomposition
The seal is vital for the subsequent thermal decomposition stage. It ensures that as the ampoule is heated, the sublimated precursor remains trapped within the system, forcing it to interact with the carbon support rather than escaping.
The Result: Deep Pore Penetration
Gas-Phase Diffusion
Because the vacuum allows the precursor to travel as a gas, the palladium possesses high mobility. This allows it to diffuse effectively throughout the container.
Internal Deposition
Unlike liquid methods that might only coat the exterior, the gas-phase precursor can navigate complex structures. It deposits palladium directly into the internal pores of the carbon support, maximizing the surface area and effectiveness of the final material.
Understanding the Trade-offs
Critical Dependence on Seal Integrity
The entire process relies on the perfection of the flame seal. If the seal is imperfect, the vacuum is lost, the sublimation temperature rises, and air interference returns, rendering the process ineffective.
Complexity of Setup
Using high-vacuum equipment and flame sealing adds a layer of operational complexity compared to simple wet-chemistry methods. It requires precise control to hit the 3.5 x 10^-2 mbar target accurately before sealing.
Making the Right Choice for Your Goal
To ensure the success of your gas-phase deposition process, consider the following focus areas:
- If your primary focus is material purity: Ensure your vacuum pump is calibrated to consistently reach or exceed the 3.5 x 10^-2 mbar threshold to remove all air interference.
- If your primary focus is maximizing catalytic surface area: Prioritize the integrity of the flame seal to maintain the conditions necessary for the gas to penetrate the internal pores of the support.
Mastering the vacuum and sealing stages is not just a preparatory step; it is the defining factor in achieving deep, uniform palladium deposition.
Summary Table:
| Component | Primary Function | Impact on Deposition Process |
|---|---|---|
| Vacuum Pump | Lowers pressure to ~3.5 x 10^-2 mbar | Triggers low-temp sublimation and removes atmospheric air |
| Flame Sealing | Hermetically closes the reaction ampoule | Maintains vacuum integrity and enables trapped thermal decomposition |
| Gas Phase | Facilitates high molecular mobility | Ensures palladium penetrates deep internal pores of carbon support |
Elevate Your Material Research with KINTEK
Precision in gas-phase deposition starts with the right equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, Tube, and Muffle furnace systems specifically designed for demanding laboratory processes. Whether you are performing palladium sublimation or complex thermal decomposition, our customizable systems ensure the stable environment you need for superior material purity and uniform deposition.
Ready to optimize your lab's high-temperature processes? Contact KINTEK experts today to find the perfect solution for your unique research needs.
Visual Guide
References
- Sarah L. Boyall, Thomas W. Chamberlain. Palladium nanoparticle deposition on spherical carbon supports for heterogeneous catalysis in continuous flow. DOI: 10.1039/d3cy01718d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the functions of alumina crucibles and quartz sleeve encapsulation in the synthesis of calcium perrhenates?
- What are the specific functions of the grinder and laboratory oven during sugarcane-based activated carbon preparation?
- How does an automatic temperature control system affect bio-char? Engineer Precise Energy Density & Pore Structure
- What role does a molecular pump set play in an electric current-assisted TLP bonding system? Enhance Vacuum Purity
- What is the function of high-vacuum encapsulated quartz tubes for Ce2(Fe, Co)17? Ensure Phase Purity and Stability
- What is the significance of high-precision mass flow controllers in testing NiFe2O4? Ensure Data Integrity
- What are the main types of laboratory furnaces? Find Your Perfect High-Temperature Solution
- How does surface finish impact the performance of alumina ceramic furnace tubes? Boost Purity and Efficiency



















