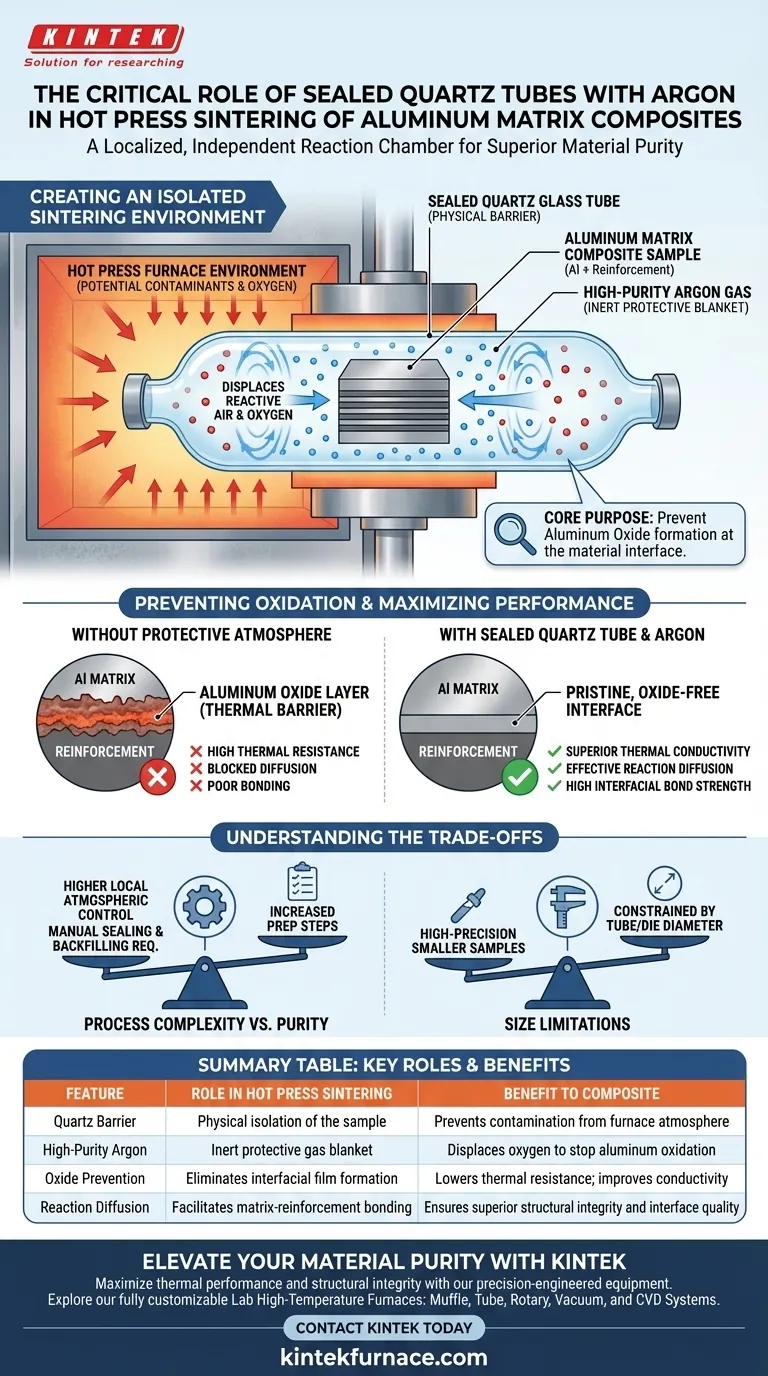
The sealed quartz glass tube functions as a localized, independent reaction chamber. By encapsulating the materials within this tube and filling it with high-purity argon, you create a stable, inert protective atmosphere that is physically isolated from the general furnace environment. This setup is the primary defense mechanism against the oxidation of high-activity aluminum alloy powders during the high-temperature sintering process.
The core purpose of this configuration is to prevent the formation of aluminum oxide layers at the material interface. By eliminating oxygen exposure, you remove the high thermal resistance associated with oxidation, ensuring superior thermal conductivity and interface quality in the final composite.

Creating an Isolated Sintering Environment
The Role of the Quartz Barrier
The quartz glass tube serves as a physical containment vessel. It acts as an independent chamber effectively separating the composite sample from the ambient atmosphere of the hot press furnace.
This isolation is critical because standard furnace environments may not be sufficiently pure. The tube ensures that the immediate environment surrounding the sample is controlled and consistent.
The Function of High-Purity Argon
Argon is introduced into the sealed tube to displace reactive air. Being an inert gas, argon does not react with the matrix or the reinforcement materials, even at elevated temperatures.
This gas acts as a "blanket," occupying the space around the powder particles. It denies oxygen the physical access needed to bond with the metal.
Preventing Oxidation and Thermal Resistance
Controlling Aluminum Reactivity
Aluminum alloy powder is classified as "high-activity." It has a strong chemical affinity for oxygen and will readily oxidize if exposed to air during heating.
Without the protective argon atmosphere, the aluminum surface would rapidly degrade. The sealed tube prevents this chemical reaction from initiating.
Eliminating Interfacial Thermal Barriers
The primary threat to aluminum matrix composites is the formation of an oxide film (aluminum oxide). As noted in the analysis of vacuum systems, these oxide films are detrimental because they create high thermal resistance.
If an oxide layer forms between the aluminum matrix and the reinforcement (such as diamond or titanium), it acts as an insulator. This hinders heat transfer and significantly reduces the thermal conductivity of the composite.
Ensuring Effective Reaction Diffusion
For the composite to have structural integrity, there must be effective diffusion between the matrix and the reinforcement. An oxide layer blocks this diffusion.
By maintaining a pristine, oxide-free environment, the quartz tube setup facilitates a high-quality interface. This allows the aluminum to properly bond with the reinforcement material.
Understanding the Trade-offs
Process Complexity vs. Purity
Using a sealed quartz tube adds a manual step to the preparation process compared to open sintering. It requires careful sealing and gas backfilling.
However, this complexity yields a higher degree of local atmospheric control. It is often more effective at protecting sensitive samples than relying solely on the vacuum level of a large furnace chamber.
Size Limitations
The use of a quartz tube imposes physical constraints on the sample size. The dimensions of the composite are limited by the diameter of the available quartz tubing and the hot press die.
This method is best suited for high-precision, smaller-scale samples where material purity is the paramount concern.
Making the Right Choice for Your Project
To determine if this setup is required for your specific application, consider your performance targets:
- If your primary focus is maximizing thermal conductivity: You must use the sealed tube and argon to prevent the formation of thermally resistive oxide layers at the interface.
- If your primary focus is interfacial bond strength: The inert atmosphere is essential to prevent oxidation from blocking the reaction diffusion between the matrix and reinforcement.
Ultimately, the sealed quartz tube is not just a container; it is a critical process control tool that guarantees the purity and performance of the aluminum interface.
Summary Table:
| Feature | Role in Hot Press Sintering | Benefit to Composite |
|---|---|---|
| Quartz Barrier | Physical isolation of the sample | Prevents contamination from furnace atmosphere |
| High-Purity Argon | Inert protective gas blanket | Displaces oxygen to stop aluminum oxidation |
| Oxide Prevention | Eliminates interfacial film formation | Lowers thermal resistance; improves conductivity |
| Reaction Diffusion | Facilitates matrix-reinforcement bonding | Ensures superior structural integrity and interface quality |
Elevate Your Material Purity with KINTEK
Maximize the thermal performance and structural integrity of your aluminum matrix composites with precision-engineered equipment. Backed by expert R&D and manufacturing, KINTEK offers a wide range of lab high-temperature furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique sintering requirements.
Don't let oxidation compromise your research. Contact KINTEK today to discover how our advanced heating solutions can optimize your lab's efficiency and material quality.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How does the heating mechanism of a vacuum hot pressing sintering furnace differ from SPS? A Comparative Guide
- What is the role of HIP equipment in the diffusion bonding of 6061 aluminum alloy? Achieve High-Integrity Metallurgy
- What physical conditions are provided by the heating plate and high-voltage DC power supply? Mastery of Anodic Bonding
- What role does Hexagonal Boron Nitride (h-BN) coating play in SPS molds? Protect Your Tooling and Purity
- How does hot pressing improve mechanical properties of materials? Achieve Superior Strength and Durability
- What role does a vacuum hot pressing furnace play in (Ti2AlC + Al2O3)p/TiAl fabrication? Achieve 100% Densification
- What is the principle of hot pressing in manufacturing? Achieve High-Density Components with Precision
- How does vacuum pressure control in an SPS furnace influence cemented carbide? Achieve High-Density Sintering Success



















