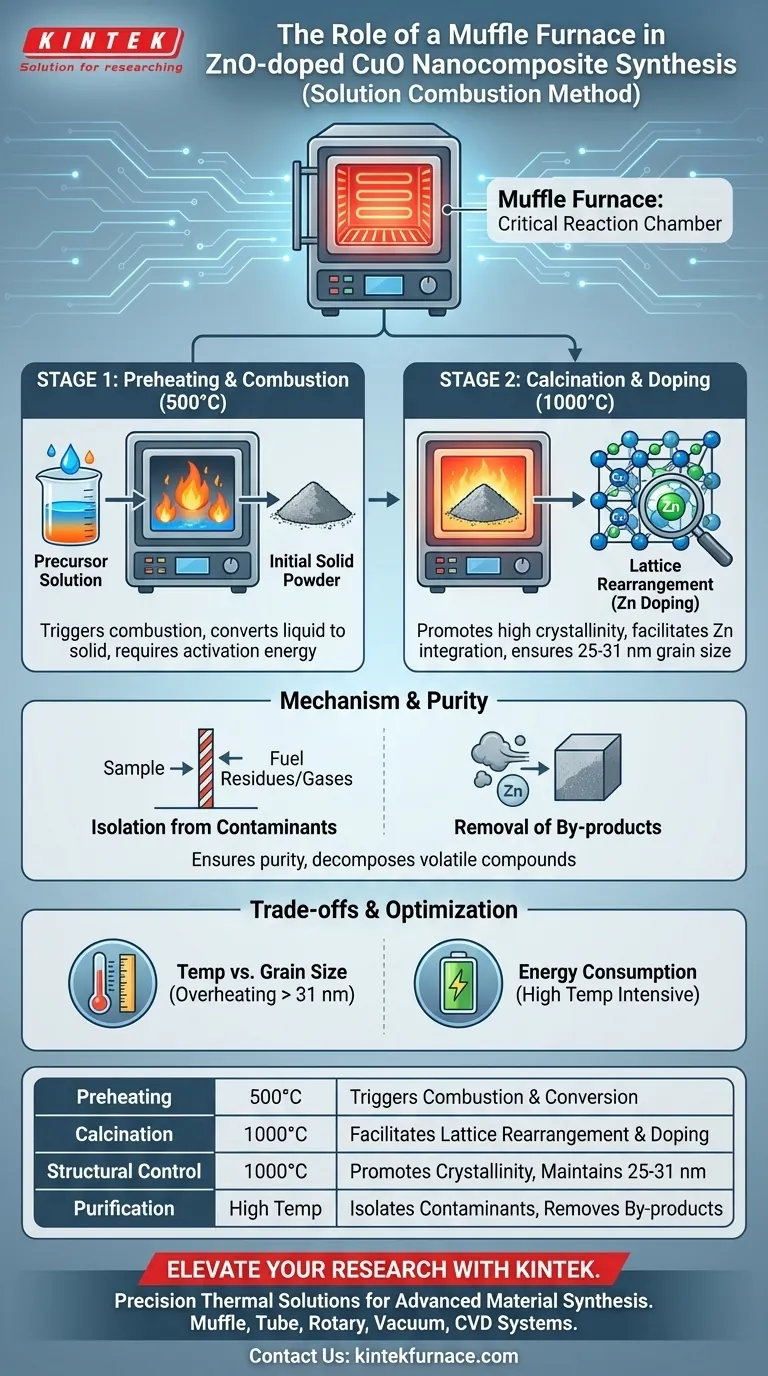
The muffle furnace acts as the critical reaction chamber for synthesizing ZnO-doped CuO nanocomposites via the solution combustion method. It provides a precisely isolated, high-temperature environment—specifically targeting 500°C for preheating and 1000°C for calcination—to drive the chemical transformation from raw precursors to a finished nanomaterial.
By delivering controlled thermal energy, the muffle furnace ensures the complete removal of combustion by-products and forces the necessary lattice rearrangement. This thermal treatment is what allows zinc to successfully dope into the copper oxide structure, yielding high crystallinity and specific grain sizes between 25 and 31 nm.

Driving the Chemical Transformation
Triggering the Combustion Reaction
The initial role of the muffle furnace is to provide the activation energy required to start the reaction. By preheating the solution to approximately 500°C, the furnace triggers the combustion process.
This rapid heating causes the solution to ignite, consuming the fuel and oxidizer. This step converts the liquid precursors into a solid, albeit initially impure, powder.
Achieving High Crystallinity
After the initial combustion, the material enters a calcination phase at a significantly higher temperature, typically 1000°C. The muffle furnace maintains this intense heat to promote crystal growth.
Without this sustained high-temperature phase, the material would remain amorphous or poorly structured. The thermal energy allows atoms to migrate and settle into a highly ordered crystalline lattice.
Facilitating Lattice Rearrangement
The specific goal of this synthesis is doping—inserting Zinc (Zn) ions into the Copper Oxide (CuO) lattice. The 1000°C environment provided by the furnace is essential for lattice rearrangement.
This process integrates the dopant ions effectively. It results in a stable nanocomposite structure rather than a simple physical mixture of two separate oxides.
Mechanism of Action and Purity
Isolation from Contaminants
A defining feature of a muffle furnace is its ability to separate the object being heated from the by-products of the heat source itself.
In the context of nanocomposites, this ensures that the ZnO-doped CuO is not contaminated by fuel residues or gases from the heating elements. This isolation is vital for maintaining the chemical purity required for semiconductor or catalytic applications.
Removal of By-products
The solution combustion method inherently produces volatile by-products. The high-temperature calcination phase effectively burns off these residues.
The furnace environment ensures that any remaining organic compounds or nitrates are completely decomposed. This leaves behind a pure oxide material with optimized electrical and structural properties.
Understanding the Trade-offs
Temperature vs. Grain Size
While high temperatures are necessary for crystallinity and doping, they also induce grain growth.
If the furnace temperature exceeds the optimal range or the dwell time is too long, the grains may grow beyond the target nanometer range. In this specific synthesis, the goal is a tight range of 25 to 31 nm; overheating effectively destroys the "nano" advantage of the material.
Energy Consumption
Muffle furnaces are energy-intensive devices, particularly when operating at 1000°C.
For large-scale production, the energy cost of this calcination step is significant. Operators must balance the need for high crystallinity against the energy efficiency of the synthesis cycle.
Making the Right Choice for Your Goal
To maximize the quality of your ZnO-doped CuO nanocomposites, you must tailor the furnace parameters to your specific objectives.
- If your primary focus is Structural Integrity and Doping: Prioritize the 1000°C calcination phase to ensure complete lattice rearrangement and successful Zinc integration.
- If your primary focus is Grain Size Control: Strictly monitor the calcination duration to prevent the particles from exceeding the 25–31 nm range, which would compromise the surface area.
Success in this synthesis relies on using the muffle furnace not just as a heater, but as a precision tool for controlling atomic-level structure.
Summary Table:
| Synthesis Phase | Temperature | Key Function of Muffle Furnace |
|---|---|---|
| Preheating | 500°C | Triggers combustion and converts liquid precursors to solid powder |
| Calcination | 1000°C | Facilitates lattice rearrangement for successful Zinc doping |
| Structural Control | 1000°C | Promotes high crystallinity while maintaining 25-31 nm grain size |
| Purification | High Temp | Isolates materials from contaminants and removes volatile by-products |
Elevate Your Material Research with KINTEK
Precision is the difference between a simple oxide and a high-performance nanocomposite. KINTEK provides industry-leading thermal solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, engineered for the rigorous demands of advanced material synthesis.
Whether you are synthesizing ZnO-doped CuO or developing next-generation semiconductors, our lab high-temperature furnaces are fully customizable to meet your specific temperature and atmospheric requirements. Backed by expert R&D and manufacturing, we ensure your lab achieves the thermal stability needed for consistent 25-31 nm grain control.
Ready to optimize your synthesis process? Contact us today to find your custom furnace solution!
Visual Guide
References
- A. Naveen Kumar, Nithesh Naik. Solution combustion synthesis of ZnO doped CuO nanocomposite for photocatalytic and sensor applications. DOI: 10.1038/s41598-024-82764-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What features might advanced muffle furnace models include? Enhance Precision, Safety, and Efficiency
- What are the primary applications of muffle furnaces? Essential for Material Analysis and Heat Treatment
- What are the advantages of muffle furnaces in terms of energy efficiency and heating speed? Achieve Fast, Efficient Heat Processing
- What role does a muffle furnace play in biochar synthesis? Expert Insights on Pulse-Based Biomass Carbonization
- What are the applications of batch furnace? Achieve Precise Thermal Processing for Your Unique Materials
- What is the primary function of a high-temperature box resistance furnace? Optimize Superalloy Homogenization
- What are the energy-saving features in modern muffle furnaces? Boost Efficiency and Cut Costs in Your Lab
- What are the cost differences between industrial muffle furnaces and drying ovens? Understand the Price Gap and Choose Wisely



















