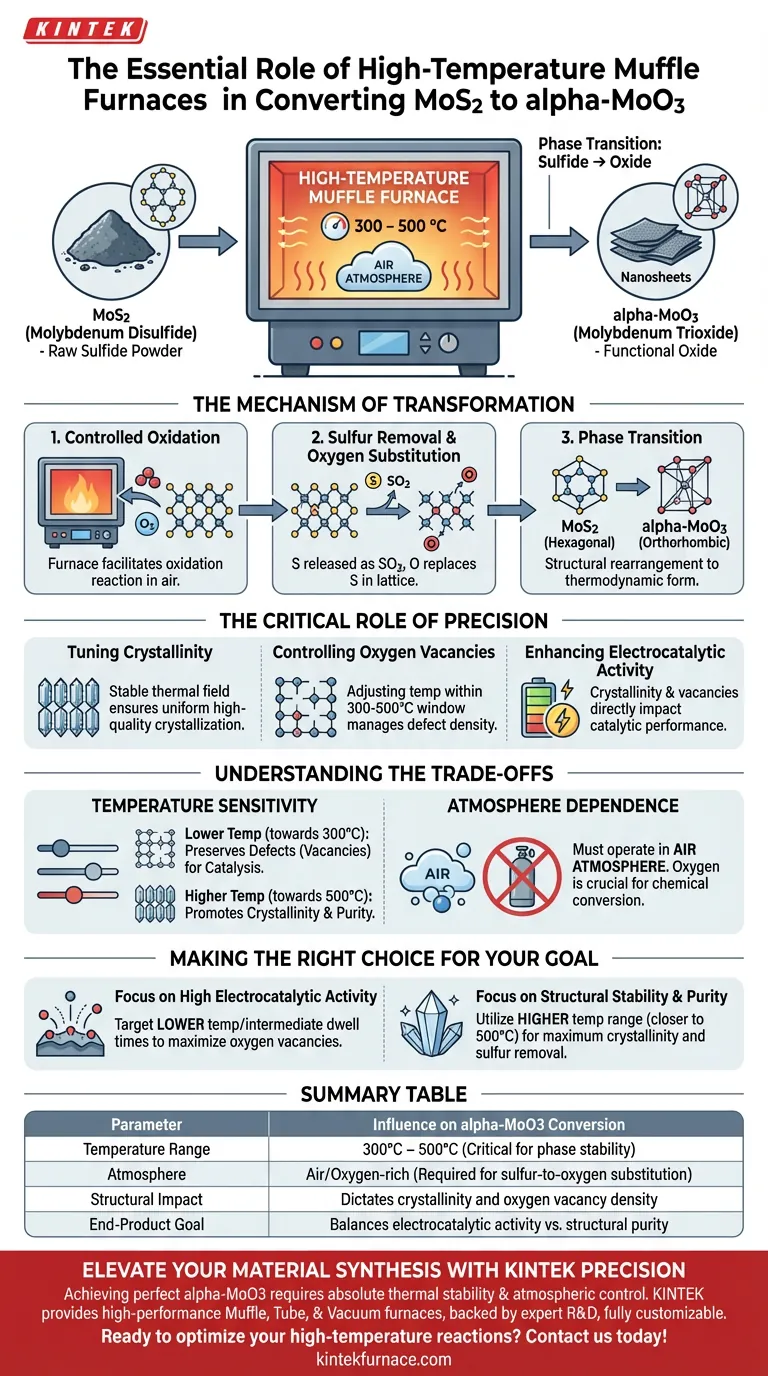
A high-temperature muffle furnace serves as the precise reaction vessel for the chemical oxidation required to convert molybdenum disulfide (MoS2) into alpha-molybdenum trioxide (alpha-MoO3). By subjecting the material to a controlled temperature range of 300 to 500 °C in an air atmosphere, the furnace drives a calcination process that systematically removes sulfur and introduces oxygen. This results in a complete phase transition from the sulfide structure to the orthorhombic oxide structure.
The muffle furnace provides more than just heat; it delivers the thermal stability required to tune the material's atomic structure. The precision of this thermal treatment directly dictates the crystallinity, defect density (oxygen vacancies), and ultimately the electrocatalytic performance of the resulting MoO3 nanosheets.

The Mechanism of Transformation
Controlled Oxidation
The primary function of the furnace is to facilitate an oxidation reaction. Under the heat of the furnace in an air environment, oxygen molecules interact with the MoS2 lattice.
Sulfur Removal and Oxygen Substitution
As the reaction progresses, sulfur atoms are liberated from the material, likely as sulfur dioxide gas. Simultaneously, oxygen atoms are incorporated into the lattice structure.
Phase Transition
This chemical exchange forces a structural rearrangement. The material shifts from the hexagonal structure of MoS2 to the thermodynamically distinct orthorhombic structure of alpha-MoO3.
The Critical Role of Precision
Tuning Crystallinity
The exact temperature maintained by the muffle furnace determines how ordered the final crystal structure becomes. A stable thermal field ensures uniform energy distribution, leading to consistent high-quality crystallization across the sample.
Controlling Oxygen Vacancies
One of the most nuanced roles of the furnace is manipulating oxygen vacancy concentrations. By adjusting the specific calcination temperature within the 300–500 °C window, you can control the density of these atomic defects.
Enhancing Electrocatalytic Activity
The physical properties derived from the heat treatment—specifically the crystallinity and vacancy concentration—directly impact the material's function. A precisely executed furnace cycle yields MoO3 nanosheets with optimized electrocatalytic activity.
Understanding the Trade-offs
Temperature Sensitivity
While the furnace enables transformation, the specific temperature chosen involves a trade-off. Lower temperatures in the range may preserve more defects (vacancies) which can be beneficial for catalysis, while higher temperatures typically promote higher crystallinity but may reduce these active sites.
Atmosphere Dependence
The muffle furnace must operate with an air atmosphere for this specific conversion. Unlike inert gas sintering used for other materials, this process relies on the availability of atmospheric oxygen to drive the chemical conversion from sulfide to oxide.
Making the Right Choice for Your Goal
To maximize the utility of the MoS2 to alpha-MoO3 conversion, you must tailor the furnace parameters to your specific end-goal.
- If your primary focus is high electrocatalytic activity: Target the lower end of the temperature spectrum or specific intermediate dwell times to maximize oxygen vacancy concentrations, which often act as active sites.
- If your primary focus is structural stability and purity: Utilize the higher end of the temperature range (closer to 500 °C) to ensure maximum crystallinity and the complete removal of all sulfur residues.
By strictly controlling the thermal profile, the muffle furnace transforms a raw sulfide powder into a highly tunable functional oxide.
Summary Table:
| Parameter | Influence on alpha-MoO3 Conversion |
|---|---|
| Temperature Range | 300°C – 500°C (Critical for phase stability) |
| Atmosphere | Air/Oxygen-rich (Required for sulfur-to-oxygen substitution) |
| Structural Impact | Dictates crystallinity and oxygen vacancy density |
| End-Product Goal | Balances electrocatalytic activity vs. structural purity |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect alpha-MoO3 phase transition requires more than just heat—it demands absolute thermal stability and atmospheric control. At KINTEK, we empower researchers and manufacturers with high-performance Muffle, Tube, and Vacuum furnaces specifically designed for sensitive calcination and oxidation processes.
Our systems are backed by expert R&D and are fully customizable to meet your unique laboratory or industrial needs. Whether you are tuning oxygen vacancies for catalysis or ensuring high-purity crystallization, KINTEK provides the reliability you need to succeed.
Ready to optimize your high-temperature reactions? Contact us today to discuss your custom furnace requirements!
Visual Guide
References
- Electrocatalytic Hydrogen Generation from Seawater at Neutral pH on a Corrosion-Resistant MoO<sub>3</sub>/Ti-Felt Electrode. DOI: 10.1021/acssuschemeng.5c02839
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What process conditions must a muffle furnace satisfy for CoNiCrAlY oxidation? Ensure Precise High-Temp Stability
- What is the significance of box type electric furnaces in metal melting? Precision Control for Small-Scale Metallurgy
- What is the maximum temperature of the muffle furnace described? Key Limits for Lab Success
- What is the role of a high-temperature muffle furnace in solid-state synthesis? Master CaMnO3 Perovskite Production
- How does a muffle furnace compare to other high-temperature furnaces in terms of cost? Discover Affordable Heat Treatment Solutions
- What is the significance of muffle furnaces in materials science? Unlock Pure, High-Temperature Processing
- How does a muffle furnace control the atmosphere around the sample? Achieve Precise Material Processing
- How does a muffle furnace protect samples from contamination? Ensure Purity with Advanced Isolation



















