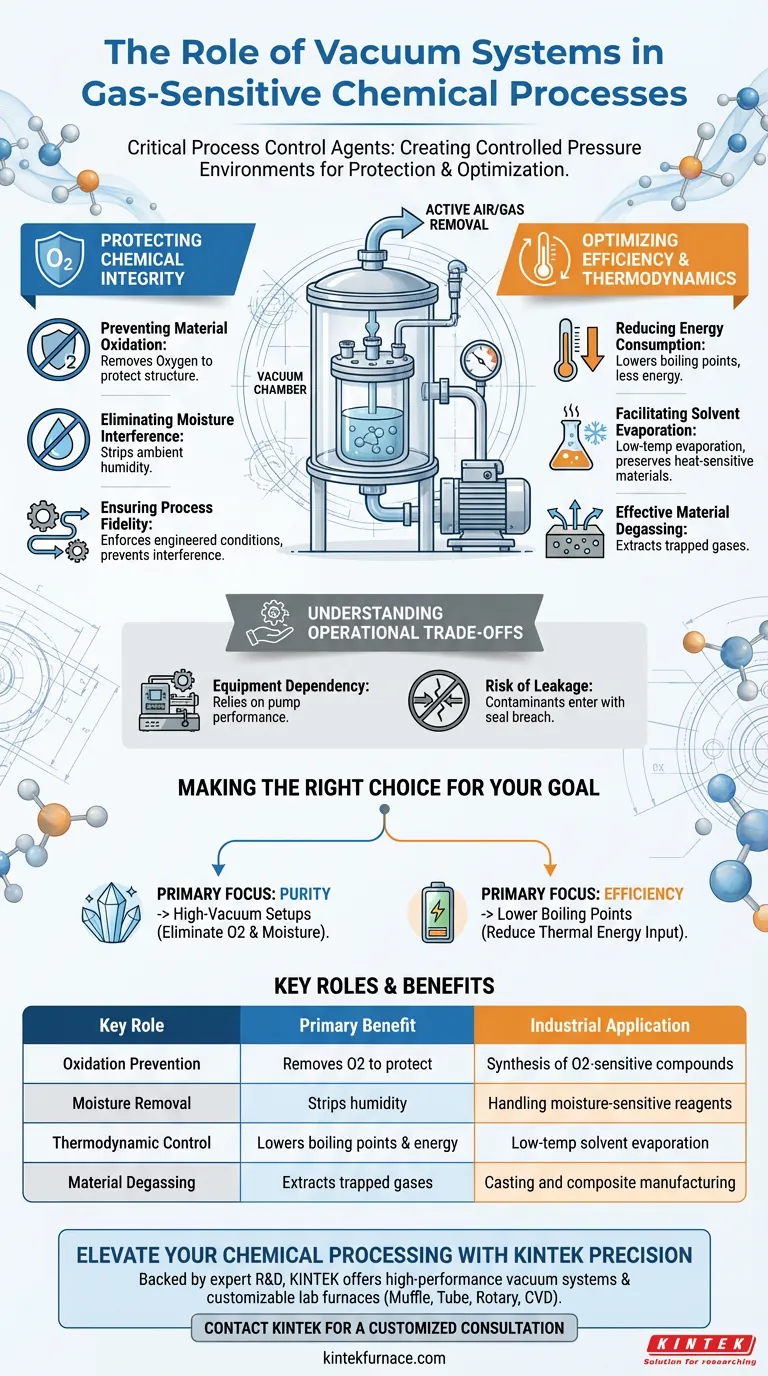
Vacuum systems act as critical process control agents in complex chemical environments. Their primary function is to actively remove air or specific gases to create a controlled pressure environment, which is essential for preventing material degradation and optimizing the thermodynamic conditions of a reaction.
By removing atmospheric interference, vacuum systems effectively shield sensitive compounds from oxidation and significantly reduce the thermal energy required to drive chemical processes.
Protecting Chemical Integrity
Preventing Material Oxidation
For syntheses involving oxygen-sensitive compounds, the presence of standard air is detrimental. Vacuum systems evacuate the reaction vessel to remove oxygen, effectively preventing material oxidation and ensuring the chemical structure remains intact.
Eliminating Moisture Interference
Many chemical reactions are highly sensitive to moisture found in the atmosphere. By lowering the pressure and removing air, vacuum systems also strip away ambient humidity, protecting moisture-sensitive reagents from unwanted side reactions.
Ensuring Process Fidelity
Chemical processes often require a specific, "predefined path" to yield the correct result. Vacuum systems enforce the necessary physical and chemical conditions, ensuring the reaction proceeds exactly as engineered without interference from environmental variables.
Optimizing Efficiency and Thermodynamics
Reducing Energy Consumption
One of the most tangible benefits of a vacuum environment is the reduction of boiling points. By lowering the system pressure, you reduce the energy required to drive phase changes, such as evaporation.
Facilitating Solvent Evaporation
In processes requiring the removal of solvents, a vacuum allows evaporation to occur at much lower temperatures. This is critical for preserving heat-sensitive materials that might degrade under the high heat required at atmospheric pressure.
Effective Material Degassing
Trapped gases within a material can compromise its final properties. Vacuum systems provide the negative pressure needed to pull these trapped gases out of the bulk material, a process known as material degassing.
Understanding the Operational Trade-offs
Equipment Dependency
Relying on vacuum systems introduces a layer of mechanical complexity. The process becomes entirely dependent on the pump's ability to maintain the "controlled pressure environment."
Risk of Leakage
Because the system operates below atmospheric pressure, any breach in the seal invites contaminants in, rather than letting material out. A minor leak can reintroduce oxygen or moisture, immediately compromising the predefined path of the reaction.
Making the Right Choice for Your Goal
To determine how to best leverage vacuum technology in your specific process, consider your primary constraints:
- If your primary focus is Purity: Utilize high-vacuum setups to completely eliminate oxygen and moisture, preventing oxidation and side reactions.
- If your primary focus is Efficiency: Implement vacuum systems to lower solvent boiling points, significantly reducing the thermal energy input required for evaporation.
Vacuum systems are not merely about removing air; they are a tool for precision engineering the physical environment to guarantee chemical success.
Summary Table:
| Key Role | Primary Benefit | Industrial Application |
|---|---|---|
| Oxidation Prevention | Removes O2 to protect chemical structures | Synthesis of oxygen-sensitive compounds |
| Moisture Removal | Strips ambient humidity to stop side reactions | Handling of moisture-sensitive reagents |
| Thermodynamic Control | Lowers boiling points & energy consumption | Low-temp solvent evaporation |
| Material Degassing | Extracts trapped gases from bulk materials | Casting and composite manufacturing |
Elevate Your Chemical Processing with KINTEK Precision
Don't let atmospheric interference compromise your material integrity. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems and customizable lab high-temp furnaces—including Muffle, Tube, Rotary, and CVD systems—engineered to meet your most demanding environmental requirements. Whether you need to prevent oxidation or optimize thermal efficiency, our technical team is ready to design the perfect solution for your lab.
Ready to achieve superior process fidelity? Contact KINTEK today for a customized consultation!
Visual Guide
References
- Mithun Prakash Ravikumar, Sakar Mohan. Iron Nitride‐Derived In Situ <i>N</i>‐doped Fe<sub>2</sub>O<sub>3</sub> Nanoaggregates with Optimized Band Structure for Solar‐Driven Photocatalytic Water Splitting. DOI: 10.1002/asia.202500484
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Vacuum Heat Treat Sintering and Brazing Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
People Also Ask
- How does vacuum compare to other atmosphere control methods? Achieve Superior Purity and Simplicity
- What are the advantages of using a vacuum heating furnace for SAE52100 steel? Maximize Hardness & Surface Integrity
- How are vacuum furnaces environmentally friendly? Achieve Clean, Efficient Heat Treatment
- How does a vacuum furnace prevent oxidation of metals? Unlock Purity and Strength in Heat Treatment
- What are the primary reasons for using movable material baskets to load scrap magnesium shavings into a vacuum sublimation furnace? Maximize Efficiency & Safety
- What are the advantages of a mesh belt brazing furnace vs vacuum? Optimize High-Volume Stainless Steel Production
- What are the key features of vacuum furnaces? Achieve Absolute Control for High-Performance Materials
- What are the types of vacuum furnaces based on heating form? Internal vs. External Heating Explained



















