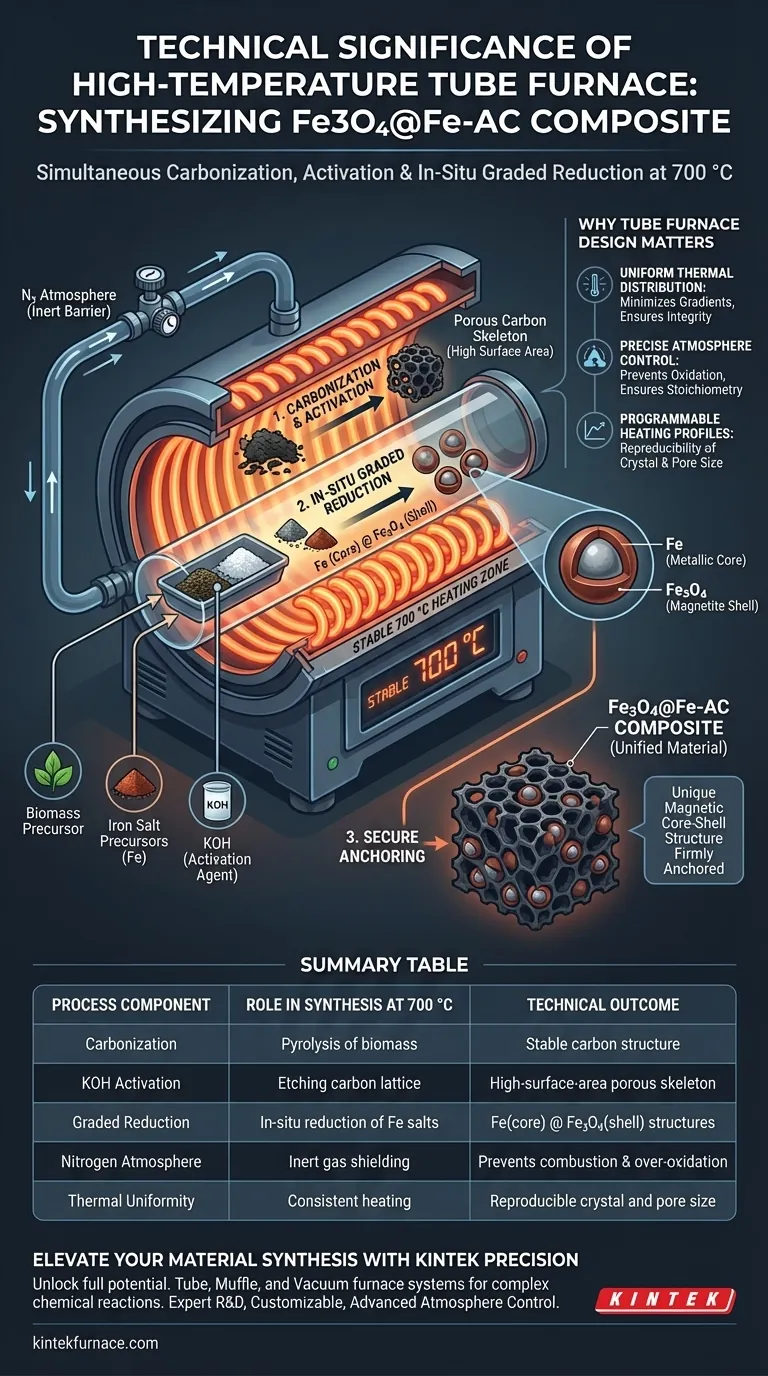
The technical significance lies in the simultaneous orchestration of carbonization, activation, and chemical reduction. A tube furnace provides the stable 700 °C, nitrogen-protected environment required to transform biomass and iron precursors into a complex Fe3O4@Fe-AC composite in a single step. Without this precise thermal envelope, the distinct magnetic core-shell structures would fail to form or anchor correctly onto the carbon skeleton.
The high-temperature environment facilitates the "graded reduction" of iron species while simultaneously creating a porous carbon skeleton, ensuring magnetic core-shell structures are securely anchored to the material.

The Mechanism of Synthesis at 700 °C
The production of Fe3O4@Fe-AC is not merely about heating materials; it is about driving specific, concurrent chemical reactions that define the material's final properties.
Simultaneous Carbonization and Activation
At 700 °C, the biomass precursor undergoes pyrolysis, converting organic matter into a stable carbon structure.
Concurrently, KOH activation occurs within this heated zone. This process etches the developing carbon lattice, generating a highly developed porous skeleton that serves as the substrate for the iron particles.
In-Situ Graded Reduction
The most critical technical function of this environment is the in-situ reduction of iron species.
Under the protection of high-purity nitrogen, iron salt precursors are not simply dried; they undergo a graded reduction. This specific thermal pathway creates a unique magnetic core-shell structure, consisting of a metallic Iron (Fe) core surrounded by a Magnetite (Fe3O4) shell.
Secure Anchoring
The high temperature ensures these magnetic structures are not loose particles but are chemically anchored to the porous carbon.
This integration prevents the leaching of magnetic components and ensures the composite acts as a unified material during application.
Why the Tube Furnace Design Matters
While the temperature drives the chemistry, the tube furnace hardware ensures the reactions occur uniformly across the sample.
Uniform Thermal Distribution
Tube furnaces are designed to minimize temperature gradients along the length of the heating zone.
This uniformity is vital for experimental integrity. It ensures that the graded reduction occurs at the same rate throughout the batch, preventing a mix of over-reduced (pure Fe) and under-reduced (oxide) particles.
Precise Atmosphere Control
The tube design allows for a sealed, continuous flow of high-purity nitrogen.
This creates an inert barrier against oxidation. If oxygen were to leak into the chamber at 700 °C, the carbon would burn off (combust) rather than graphitize, and the iron would fully oxidize rather than forming the metallic core.
Programmable Heating Profiles
Advanced controllers allow for specific ramp rates and soak times.
This control dictates the crystal growth size of the iron species and the pore size distribution of the carbon, allowing for reproducibility between synthesis batches.
Understanding the Trade-offs
While the tube furnace provides precision, it introduces specific limitations that must be managed.
Scale vs. Precision
The uniform zone in a tube furnace is spatially limited. While excellent for high-quality synthesis, scaling this process up for mass production often results in thermal gradients that degrade the core-shell structure quality.
Sensitivity to Gas Flow
The synthesis is highly sensitive to the nitrogen flow rate. Excessive flow can cool the sample surface, while insufficient flow can fail to flush evolved gases, potentially altering the reduction stoichiometry.
Making the Right Choice for Your Goal
The successful synthesis of Fe3O4@Fe-AC depends on tuning the furnace parameters to your specific performance targets.
- If your primary focus is Magnetic Strength: Prioritize strict atmosphere control and precise temperature stability to protect the metallic Fe core from oxidation.
- If your primary focus is Surface Area (Porosity): Focus on the soak time at 700 °C to allow the KOH activation to fully develop the carbon skeleton without collapsing the pores.
Ultimately, the tube furnace acts as a precision reactor that forces the simultaneous evolution of porosity and magnetism into a single, stable composite.
Summary Table:
| Process Component | Role in Synthesis at 700 °C | Technical Outcome |
|---|---|---|
| Carbonization | Pyrolysis of biomass precursors | Formation of stable carbon structure |
| KOH Activation | Chemical etching of carbon lattice | Creation of high-surface-area porous skeleton |
| Graded Reduction | In-situ reduction of iron salts | Formation of Fe (core) @ Fe3O4 (shell) structures |
| Nitrogen Atmosphere | Inert gas shielding | Prevents carbon combustion and over-oxidation |
| Thermal Uniformity | Consistent heating across sample | Ensures reproducible crystal and pore size |
Elevate Your Material Synthesis with KINTEK Precision
Unlock the full potential of your composite materials with KINTEK’s industry-leading thermal solutions. Whether you are developing Fe3O4@Fe-AC or advanced catalysts, our Tube, Muffle, and Vacuum furnace systems provide the stable thermal envelopes and precise atmosphere control essential for complex chemical reactions.
Why Choose KINTEK?
- Expert R&D & Manufacturing: Precision-engineered for uniform thermal distribution.
- Fully Customizable: Tailored systems to meet your unique laboratory or pilot-scale needs.
- Advanced Atmosphere Control: High-purity gas flow management for perfect material stoichiometry.
Ready to achieve superior results in your high-temperature research? Contact our experts today to find the perfect furnace solution for your lab!
Visual Guide
References
- Ka Chun Li, Xijun Hu. Fe<sub>3</sub>O<sub>4</sub>@Fe Core–Shell Okara-Derived Activated Carbon for Superior Polysulfide Control in Lithium–Sulfur Batteries. DOI: 10.1021/acs.jpcc.5c02606
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis



















