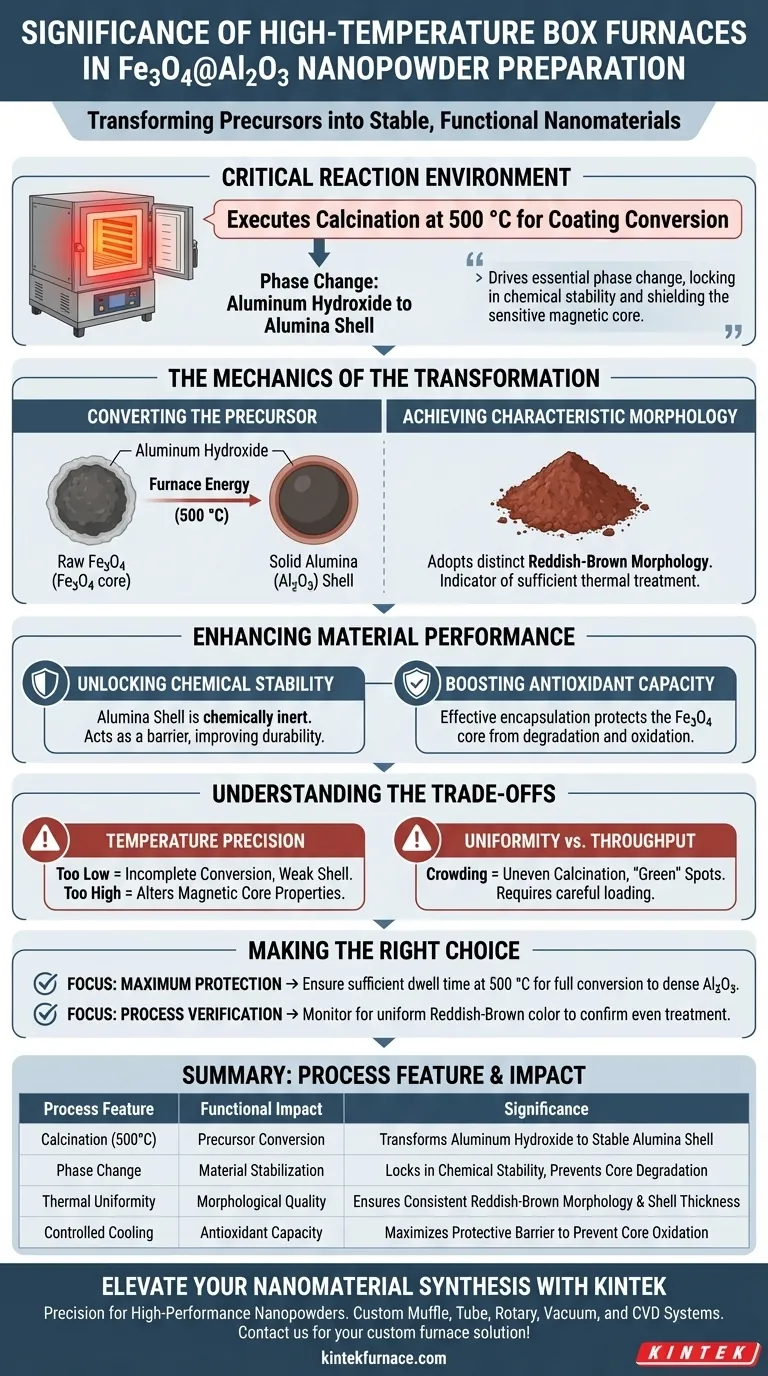
The laboratory high-temperature box furnace serves as the critical reaction environment for transforming raw precursor materials into functional Fe3O4@Al2O3 nanopowders. Specifically, it is used to execute a calcination process at temperatures around 500 °C, which is necessary to convert the coating on the magnetic core into a stable alumina shell.
By maintaining a consistent thermal environment, the furnace drives the essential phase change from aluminum hydroxide to alumina. This transformation is the key to locking in chemical stability and oxidation resistance, effectively shielding the sensitive magnetic core from degradation.

The Mechanics of the Transformation
Converting the Precursor
The primary function of the furnace in this application is to facilitate calcination.
At temperatures such as 500 °C, the furnace provides the energy required to chemically alter the precursor components. Specifically, it converts the aluminum hydroxide layer coated on the magnetic core into a solid alumina (Al2O3) shell.
Achieving Characteristic Morphology
The thermal treatment is not merely for hardening the material; it defines its physical appearance.
Following the calcination process, the nanopowder adopts a distinct reddish-brown morphology. This visual change serves as an indicator that the thermal treatment was sufficient to effect the necessary chemical changes.
Enhancing Material Performance
Unlocking Chemical Stability
The most significant outcome of using the box furnace is the enhancement of the material's durability.
The alumina shell formed during heating is chemically inert. This shell acts as a barrier, significantly improving the chemical stability of the final nanopowder compared to the untreated precursor.
Boosting Antioxidant Capacity
Protecting the core material is paramount in magnetic applications.
The thermal treatment ensures the alumina shell effectively encapsulates the Fe3O4 core. This increases the material's antioxidant capacity, preventing the magnetic core from degrading or oxidizing when exposed to external environments.
Understanding the Trade-offs
Temperature Precision
While the furnace enables the reaction, temperature control is non-negotiable.
If the temperature is too low, the conversion from aluminum hydroxide to alumina will be incomplete, resulting in a weak shell. Conversely, excessive temperatures could potentially alter the magnetic properties of the Fe3O4 core itself.
Uniformity vs. Throughput
Box furnaces are excellent for batch processing, but they require careful loading to ensure uniform heat distribution.
Crowding the furnace can lead to uneven calcination. This results in "green" (unprocessed) spots within the powder batch where the protective shell fails to form correctly.
Making the Right Choice for Your Goal
To maximize the quality of your Fe3O4@Al2O3 nanopowders, you must align your furnace settings with your specific material requirements.
- If your primary focus is maximum protection: Ensure the dwell time at 500 °C is sufficient to fully convert all aluminum hydroxide into a dense Al2O3 barrier.
- If your primary focus is process verification: Monitor the final product for a uniform reddish-brown color to confirm the thermal treatment was applied evenly throughout the batch.
The high-temperature box furnace is not just a heater; it is the tool that stabilizes your material and defines its lifespan.
Summary Table:
| Process Feature | Functional Impact | Significance in Fe3O4@Al2O3 Preparation |
|---|---|---|
| Calcination (500°C) | Precursor Conversion | Transforms aluminum hydroxide into a stable alumina (Al2O3) shell. |
| Phase Change | Material Stabilization | Locks in chemical stability and prevents magnetic core degradation. |
| Thermal Uniformity | Morphological Quality | Ensures a consistent reddish-brown morphology and uniform shell thickness. |
| Controlled Cooling | Antioxidant Capacity | Maximizes the protective barrier to prevent core oxidation. |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is the difference between a failed batch and a high-performance nanopowder. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems, engineered to deliver the exacting temperature uniformity required for Fe3O4@Al2O3 calcination.
Backed by expert R&D and manufacturing, our laboratory high-temperature furnaces are fully customizable to meet your unique research or production needs. Ensure the stability and antioxidant capacity of your materials with equipment designed for excellence.
Ready to optimize your thermal processing? Contact KINTEK today to discuss your custom furnace solution with our technical experts!
Visual Guide
References
- Behrooz Maleki, Sahar Peiman. Magnetic polymeric ionic liquid for both catalysis application and magnetic solid phase extraction approach. DOI: 10.1038/s41598-025-86751-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the role of a high-temperature muffle furnace in Mg-Zn-Al LDH transformation? Unlocking Adsorption Power
- What is the primary role of a muffle furnace in the calcination of Pt-xWO3/SiO2? Optimize Catalyst Phase-Engineering
- What factors influence the choice of a muffle furnace? Key Considerations for Optimal Lab Performance
- What task does a high-temperature box resistance furnace perform in Mg(Al1-xCrx)2O4 prep? Master Powder Calcination
- How is a laboratory box resistance furnace utilized in the heat treatment and testing of high-speed steel samples?
- How does the muffle furnace prevent run-away conditions? Ensure Safe, Reliable High-Temperature Operations
- What is the significance of using a muffle furnace for Y2O3 coated MgO: Ce3+? Optimize Particle Crystallization
- What is the primary function of a high-temperature furnace for nanocolloid study? Expert Thermal Performance Insights



















