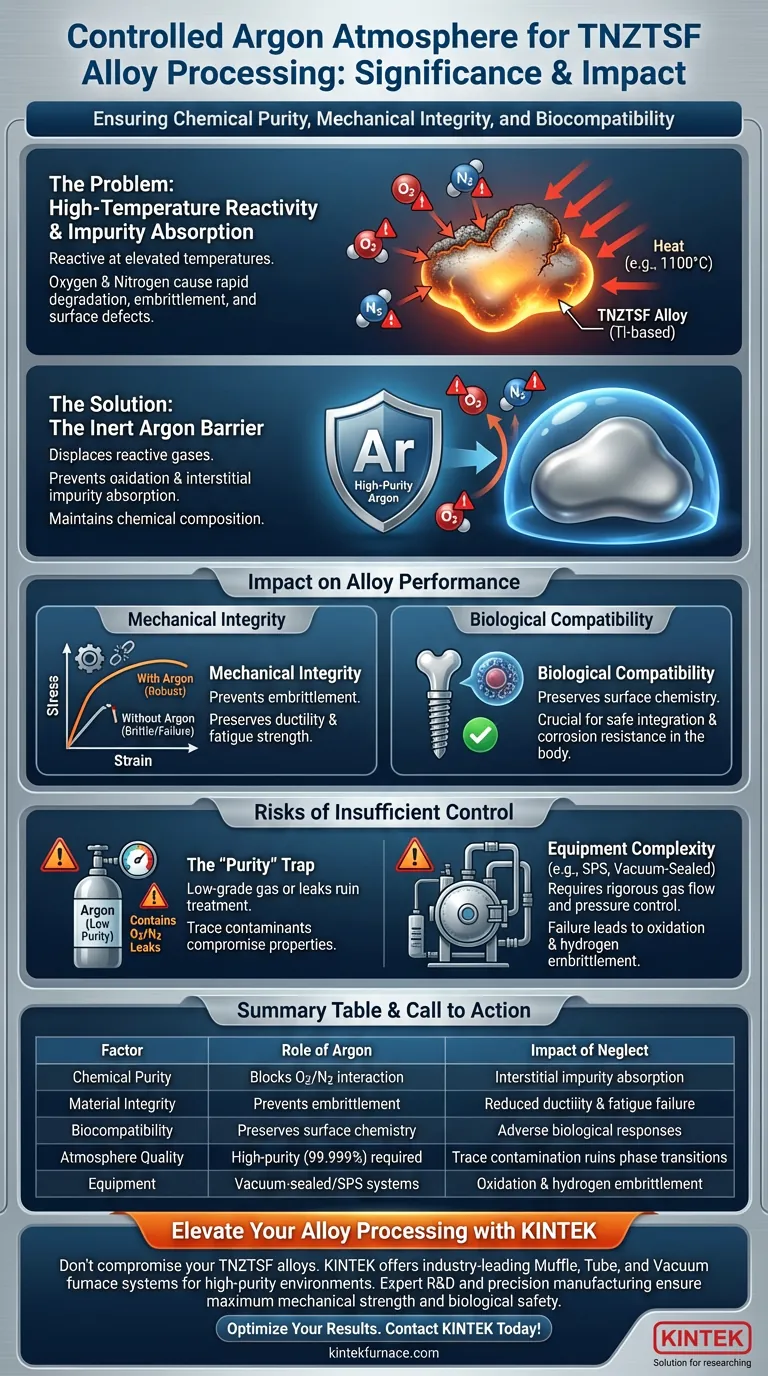
A controlled argon atmosphere acts as an essential barrier against chemical contamination. During the melting and heat treatment of TNZTSF alloys, high-purity argon isolates the material to prevent interaction with atmospheric oxygen and nitrogen. Because titanium alloys are highly reactive at elevated temperatures, this inert environment is the primary defense against oxidation and impurity absorption.
Core Takeaway: Titanium-based alloys possess a high chemical affinity for reactive gases when heated, leading to rapid degradation of material properties. Maintaining a controlled argon atmosphere is the definitive method to preserve the alloy's chemical purity, ensuring the mechanical integrity and biological compatibility required for high-performance applications.

The Critical Role of Inert Environments
Combatting High-Temperature Reactivity
TNZTSF alloys are titanium-based, making them chemically aggressive when exposed to heat.
At elevated temperatures (such as 1100°C), these alloys react readily with oxygen and nitrogen found in ambient air. A controlled argon atmosphere displaces these reactive gases, preventing them from bonding with the metal matrix.
Preventing Impurity Absorption
Beyond surface oxidation, high temperatures can cause the alloy to absorb atmospheric gases like a sponge.
This absorption introduces interstitial impurities into the metal's crystal lattice. Using an argon environment effectively blocks this absorption mechanism, maintaining the alloy's original chemical composition.
Impact on Alloy Performance
Preserving Mechanical Integrity
The intrusion of oxygen or nitrogen is not merely a surface defect; it fundamentally alters the material's mechanics.
Oxidation and gas absorption can lead to embrittlement, significantly reducing the ductility and fatigue strength of the alloy. By preventing these reactions, argon ensures the final product retains the structural robustness intended by its design.
Ensuring Biological Compatibility
For TNZTSF alloys intended for biomedical applications, purity is paramount.
Surface oxides or chemical alterations can trigger adverse biological responses or degrade the material's corrosion resistance in the body. The argon shield preserves the pristine surface chemistry necessary for safe integration with biological tissues.
Risks of Insufficient Control
The "Purity" Trap
Simply introducing argon is not enough; the gas must be of high purity (often 99.999%) to be effective.
Using low-grade argon or allowing leaks in the furnace chamber can introduce enough oxygen to ruin the heat treatment. Even trace amounts of contaminants can shift phase transformation temperatures or compromise the protective passive layer of the alloy.
Complexity of Atmosphere Systems
maintaining this environment requires sophisticated equipment, such as vacuum-sealed furnaces or Spark Plasma Sintering (SPS) systems.
These systems must rigorously control gas flow and pressure. Any failure in the containment system allows for "hydrogen embrittlement" or oxidation, rendering the processing steps futile and the material unusable.
Making the Right Choice for Your Goal
To ensure successful processing of TNZTSF alloys, evaluate your atmosphere control strategy based on your specific requirements:
- If your primary focus is Biological Safety: Prioritize ultra-high purity argon to prevent surface oxides that could compromise biocompatibility.
- If your primary focus is Mechanical Durability: rigorous atmosphere control is required to prevent interstitial embrittlement, which causes catastrophic failure under load.
Ultimately, the quality of your argon atmosphere dictates the difference between a high-performance alloy and a brittle, unusable failure.
Summary Table:
| Factor | Role of Argon Atmosphere | Impact of Neglect |
|---|---|---|
| Chemical Purity | Blocks oxygen/nitrogen interaction | Interstitial impurity absorption |
| Material Integrity | Prevents embrittlement | Reduced ductility and fatigue failure |
| Biocompatibility | Preserves surface chemistry | Adverse biological responses |
| Atmosphere Quality | High-purity gas (99.999%) required | Trace contamination ruins phase transitions |
| Equipment | Vacuum-sealed/SPS systems | Oxidation and hydrogen embrittlement |
Elevate Your Alloy Processing with KINTEK
Don’t compromise the integrity of your TNZTSF alloys with substandard atmosphere control. KINTEK provides industry-leading, customizable Muffle, Tube, and Vacuum furnace systems specifically designed for high-purity inert gas environments. Backed by expert R&D and precision manufacturing, our lab high-temp furnaces ensure your materials achieve maximum mechanical strength and biological safety.
Ready to optimize your heat treatment results? Contact KINTEK today to find the perfect furnace solution for your unique needs!
Visual Guide
References
- Vasile Dănuț Cojocaru, Bogdan Mihai Gălbinaşu. The Effect of Solution Treatment Duration on the Microstructural and Mechanical Properties of a Cold-Deformed-by-Rolling Ti-Nb-Zr-Ta-Sn-Fe Alloy. DOI: 10.3390/ma17040864
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- Is a vacuum alone sufficient to prevent oxidation in furnaces? Discover the critical role of atmosphere control
- What are the characteristics and uses of hydrogen atmosphere in furnaces? Achieve Superior Surface Purity and Bonding
- What is the purpose of a chemically reactive atmosphere in material processing? Achieve Precise Surface Modification for Enhanced Performance
- What role does an argon atmosphere play when sintering WC-Co-Ni alloys? Achieve Near-Theoretical Density
- What types of configurations are available for retort furnaces? Optimize Your Thermal Process with the Right Setup
- What advantages does the box type annealing atmosphere furnace offer? Achieve Superior Heat Treatment Control
- What types of high-temperature processes can an atmosphere furnace perform? Unlock Material Transformation with Precision Control
- What role does a high-temperature argon atmosphere furnace play? Master h-BN Interface Layer Heat Treatment



















