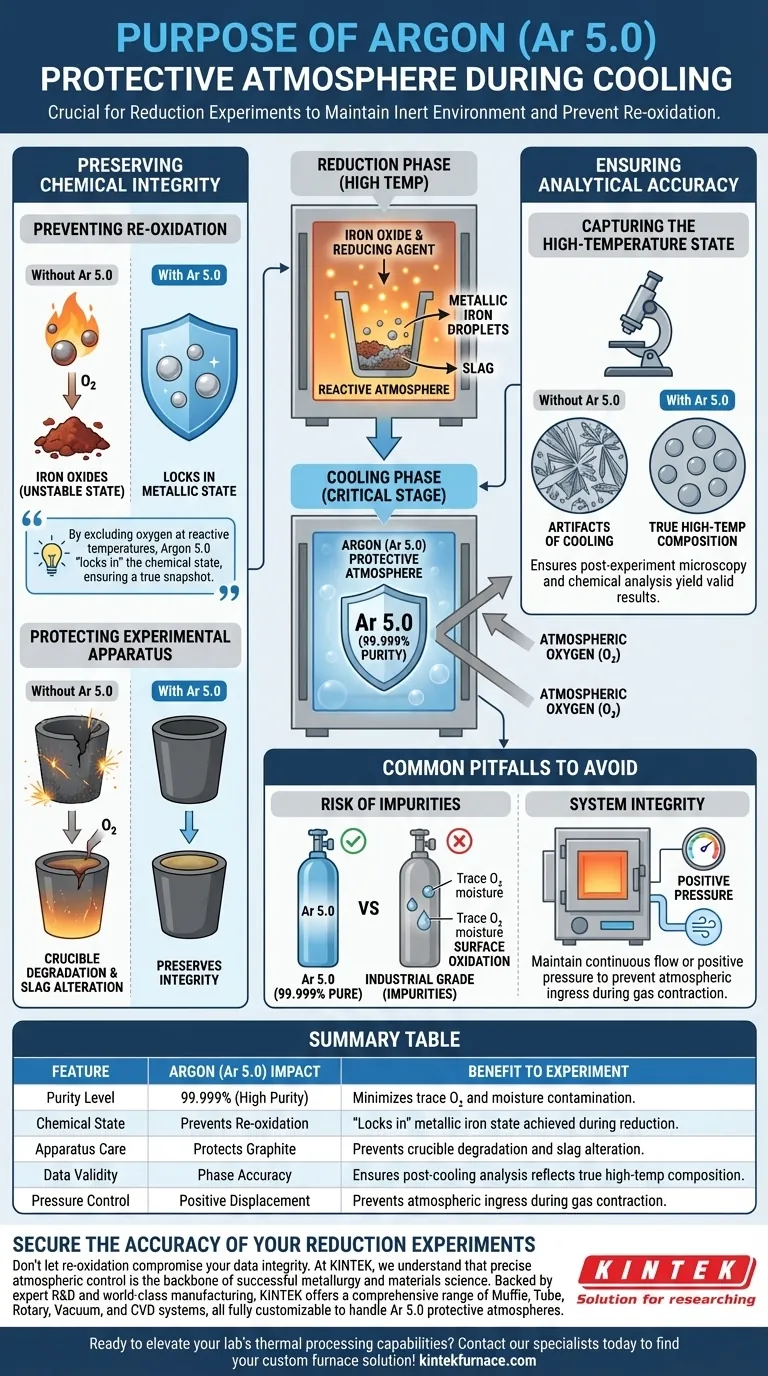
The primary purpose of using an Argon (Ar 5.0) protective atmosphere during the cooling phase is to maintain a strictly inert environment that creates a barrier against atmospheric oxygen. This prevents the newly formed metallic iron droplets from re-oxidizing, which would otherwise undo the chemical reduction achieved during the experiment.
By excluding oxygen while the sample remains at reactive temperatures, Argon 5.0 "locks in" the chemical state of the material. This ensures that the solid sample you analyze is a true snapshot of the high-temperature reduction process, rather than an artifact of the cooling conditions.

Preserving Chemical Integrity
To understand the necessity of Argon 5.0, one must recognize that chemical activity does not stop immediately when the heating elements are turned off.
Preventing Re-oxidation
During the reduction phase, you expend energy to convert iron oxides into metallic iron. This new metallic state is highly unstable in the presence of oxygen, especially at high temperatures.
Without a protective Argon atmosphere, atmospheric oxygen would aggressively react with the hot metal droplets. This reaction causes the iron to revert to an oxide state, rendering your reduction efficiency data inaccurate.
Protecting the Experimental Apparatus
The benefits of an inert atmosphere extend beyond the sample itself to the equipment containing it.
Graphite crucibles, commonly used in these experiments, are highly susceptible to oxidation. If exposed to air while hot, the graphite will react with oxygen and degrade rapidly.
Additionally, the residual slag composition can be altered by exposure to air. Argon prevents these side reactions, preserving the integrity of both the containment vessel and the slag chemistry.
Ensuring Analytical Accuracy
The ultimate goal of a reduction experiment is usually to analyze the phase composition to understand what occurred at peak temperatures.
Capturing the High-Temperature State
You need your solid samples to accurately represent the phase composition as it existed at the end of the high-temperature stage.
If the sample reacts with air during cooling, new chemical phases may form that did not exist during the actual experiment. Using high-purity Argon (99.999%) eliminates these variables, ensuring that your post-experiment microscopy and chemical analysis yield valid results.
Common Pitfalls to Avoid
While using Argon 5.0 is the standard for high-quality data, there are operational nuances that can undermine its effectiveness.
The Risk of Impurities
Not all Argon is created equal. The specific designation Ar 5.0 indicates a purity of 99.999%.
Using lower-grade Argon (such as industrial grade) can introduce trace amounts of oxygen or moisture. Even these small impurities can be sufficient to cause surface oxidation on metallic droplets or alter sensitive slag phases, compromising the precision of your data.
System Integrity
Pumping Argon into the chamber is only effective if the system is sealed against ingress.
A common oversight is failing to maintain positive pressure during the cooling cycle. As the gas inside the furnace cools, it contracts; without a continuous flow or positive pressure of Argon, the system may draw in outside air, negating the protective atmosphere.
Making the Right Choice for Your Goal
The decision to use high-purity Argon is ultimately a decision about data fidelity.
- If your primary focus is accurate phase analysis: You must use Ar 5.0 to prevent re-oxidation and ensure the microstructure reflects the high-temperature state.
- If your primary focus is equipment longevity: Maintaining the Argon flow until the system is well below reactive temperatures is critical to prevent graphite crucible degradation.
A high-purity protective atmosphere is not just a safety measure; it is a fundamental requirement for validating the success of your reduction process.
Summary Table:
| Feature | Argon (Ar 5.0) Impact | Benefit to Experiment |
|---|---|---|
| Purity Level | 99.999% (High Purity) | Minimizes trace oxygen and moisture contamination. |
| Chemical State | Prevents Re-oxidation | "Locks in" metallic iron state achieved during reduction. |
| Apparatus Care | Protects Graphite | Prevents crucible degradation and slag alteration at high temperatures. |
| Data Validity | Phase Accuracy | Ensures post-cooling analysis reflects true high-temp composition. |
| Pressure Control | Positive Displacement | Prevents atmospheric ingress during gas contraction. |
Secure the Accuracy of Your Reduction Experiments
Don't let re-oxidation compromise your data integrity. At KINTEK, we understand that precise atmospheric control is the backbone of successful metallurgy and materials science.
Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temp furnaces. All our systems are fully customizable to handle Ar 5.0 protective atmospheres, ensuring your high-purity reduction processes remain uncontaminated from start to finish.
Ready to elevate your lab's thermal processing capabilities? Contact our specialists today to find your custom furnace solution!
Visual Guide
References
- M. A. Levchenko, Olena Volkova. Reduction of Liquid Steelmaking Slag Using Hydrogen Gas as a Reductant. DOI: 10.3390/met15090984
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does the thermal treatment enhance the mechanical properties of AZO and ZnO coatings? Boost Durability & Hardness
- How does heat treatment affect the TPU encapsulation layer? Optimize Flexible Sensor Durability & Bonding
- Why are high-purity copper foils used as support substrates in phase equilibrium experiments with low SiO2 content?
- What role does a high-temperature thermal simulation system play in the dissolution of precipitates in steel?
- How does a rotary evaporator contribute to the concentration phase of TiO2 and ZrO2 pastes? Achieve Precision Viscosity
- What type of furnaces are commonly used for sintering? Choose the Right Furnace for Your Process
- How does a vacuum oven contribute to the performance of composite electrode slurries? Enhance Battery Life & Stability
- What is the significance of using high-temperature heating equipment to reach 1250°C for alloys? Stress Test Excellence



















