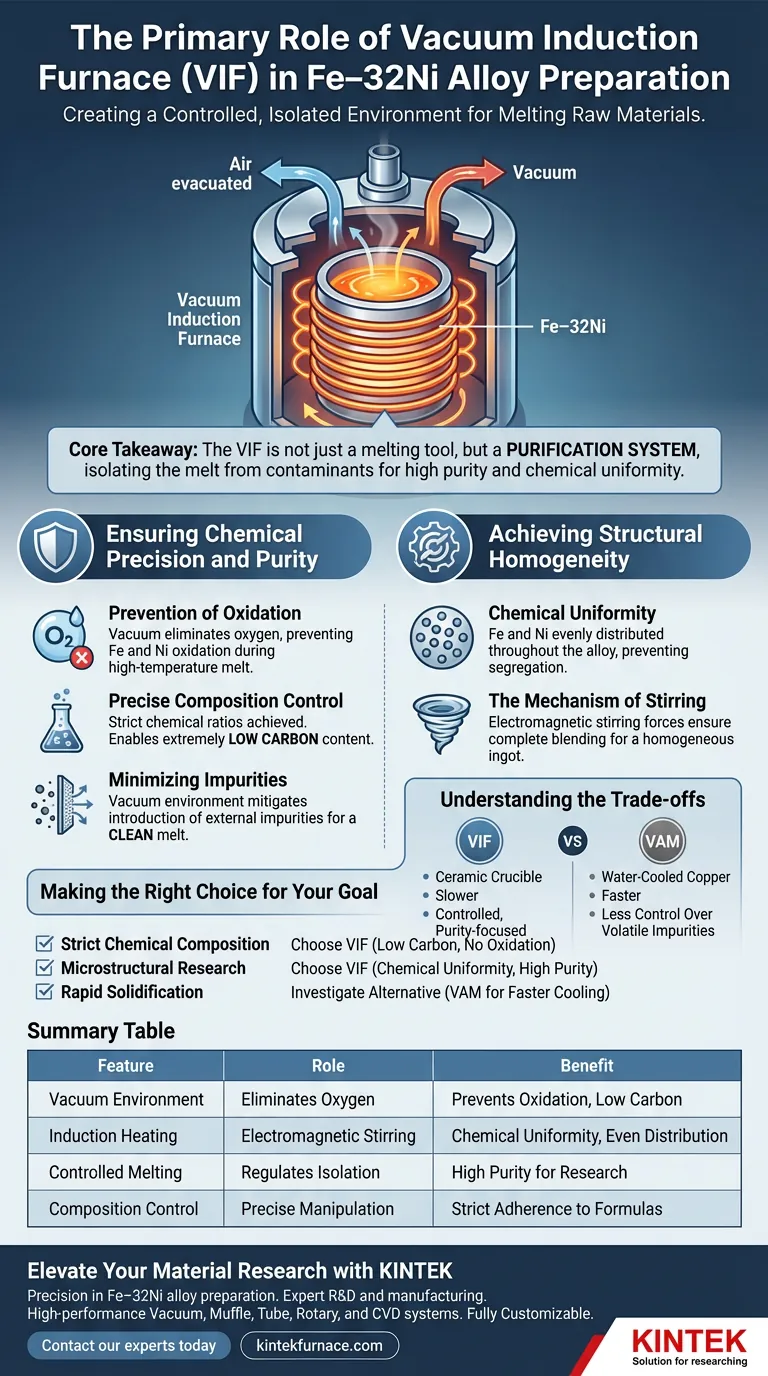
The primary role of a vacuum induction furnace (VIF) in preparing Fe–32Ni alloys is to create a controlled, isolated environment for melting raw materials. By operating under vacuum, the furnace strictly regulates the alloy's chemical composition, specifically enabling the achievement of extremely low carbon content while preventing oxidation and impurity introduction.
Core Takeaway The vacuum induction furnace is not merely a melting tool; it is a purification system. Its ability to isolate the Fe-32Ni melt from atmospheric contaminants ensures the high purity and chemical uniformity required for valid microstructural research.

Ensuring Chemical Precision and Purity
Prevention of Oxidation
The fundamental advantage of this furnace is its ability to operate in a vacuum. This effectively removes oxygen from the melting chamber.
By eliminating oxygen, the process prevents the iron and nickel from oxidizing during the high-temperature melt. This protection is critical for maintaining the integrity of the raw materials.
Precise Composition Control
Fe–32Ni alloys often require strict adherence to specific chemical ratios for research purposes. The vacuum environment allows for precise manipulation of the alloy's chemistry.
Specifically, this method is used to achieve extremely low carbon content. Without the interference of atmospheric gases, researchers can fine-tune the elemental makeup of the ingot.
Minimizing Impurities
Beyond oxidation, the vacuum environment mitigates the introduction of other external impurities.
This results in a "clean" melt, ensuring that the final ingot possesses high purity. High purity is a prerequisite for generating reliable data in subsequent microstructural analyses.
Achieving Structural Homogeneity
Chemical Uniformity
The primary reference highlights that VIF is essential for ensuring "chemical uniformity" in the resulting ingots.
This means the iron and nickel are evenly distributed throughout the alloy, rather than segregating into varying concentrations.
The Mechanism of Stirring
While the vacuum protects the chemistry, the "induction" aspect of the furnace actively mixes the alloy.
Induction heating generates electromagnetic stirring forces within the melt. This natural agitation ensures that the Fe and Ni blend completely, resulting in a homogeneous ingot.
Understanding the Trade-offs
While the vacuum induction furnace is ideal for purity and uniformity, it is important to recognize its limitations compared to other methods like Vacuum Arc Melting.
Crucible Interactions
VIFs typically use ceramic crucibles to hold the molten metal. At high temperatures, there is a risk of the melt reacting slightly with the crucible material.
This can potentially introduce non-metallic inclusions, whereas water-cooled copper crucibles (often used in arc furnaces) minimize this specific risk.
Processing Speed vs. Control
VIF is generally a slower process focused on equilibrium and control.
If the goal is rapid solidification or handling extremely refractory metals (high melting points), other furnace types might offer faster processing, though often with less control over volatile impurities.
Making the Right Choice for Your Goal
To determine if a vacuum induction furnace is the correct tool for your specific metallurgical project, consider the following:
- If your primary focus is strict chemical composition: Choose the VIF to minimize carbon content and prevent oxidation of active elements.
- If your primary focus is microstructural research: Rely on the VIF to provide the chemical uniformity and high purity necessary for accurate baselines.
- If your primary focus is rapid solidification: Investigate alternative methods like vacuum arc melting, which utilizes water-cooled crucibles for faster cooling rates.
For Fe–32Ni alloys, the vacuum induction furnace remains the standard for establishing a pristine, chemically accurate material foundation.
Summary Table:
| Feature | Role in Fe–32Ni Preparation | Benefit to Alloy |
|---|---|---|
| Vacuum Environment | Eliminates oxygen and atmospheric gases | Prevents oxidation and achieves low carbon levels |
| Induction Heating | Generates electromagnetic stirring | Ensures chemical uniformity and even Fe-Ni distribution |
| Controlled Melting | Regulates raw material isolation | High purity required for microstructural research |
| Composition Control | Precise manipulation of elemental ratios | Guarantees strict adherence to specific research formulas |
Elevate Your Material Research with KINTEK
Precision in Fe–32Ni alloy preparation starts with superior thermal control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, Muffle, Tube, Rotary, and CVD systems—all fully customizable to meet your unique laboratory requirements.
Whether you need to minimize carbon impurities or ensure perfect chemical homogeneity, our advanced high-temperature furnaces provide the reliability your research demands. Contact our experts today to discuss your specific needs and discover how our tailored solutions can optimize your metallurgy workflow.
Visual Guide
References
- Dongyun Sun, Fucheng Zhang. Effect of Cold Rolling and Cryogenic Treatment on the Microstructure and Mechanical Properties of Fe–32Ni Alloy. DOI: 10.3390/met14020174
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Why is an induction furnace equipped with a graphite susceptor necessary? Achieving Precision for Electrical Steel
- How does a high-frequency heat induction furnace contribute to the sintering of Titanium-Zirconium alloys?
- What are the core advantages of an induction furnace for magnesium powder? Achieve 20x Higher Yield
- How does IGBT enhance efficiency and energy savings in induction melting? Achieve Superior Control and Lower Costs
- What are the benefits of the compact and lightweight design of induction furnaces? Maximize Efficiency in Limited Space
- What are the primary functions of Vacuum Induction Melting (VIM) furnaces? Achieve High-Purity Metal Processing
- What is the coreless induction furnace used for in the metal thermal processing industry? Achieve Clean, Efficient Metal Melting
- What is the role of the vacuum system in a vacuum casting furnace? Ensure Purity and Performance in Metal Casting



















