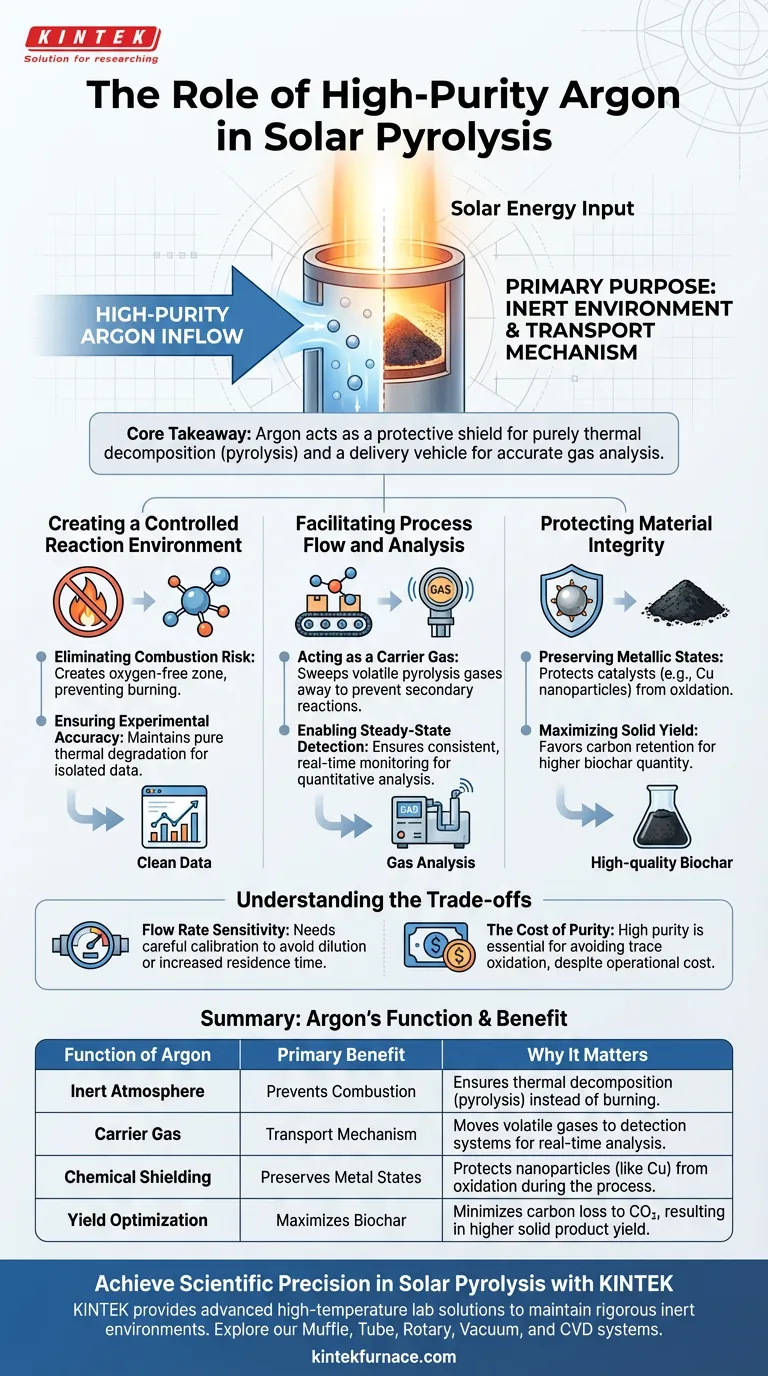
The primary purpose of continuously introducing high-purity argon gas is to establish a strictly inert environment while simultaneously acting as a transport mechanism. By displacing oxygen, argon ensures the biomass undergoes true thermal decomposition rather than combustion, while ensuring the steady movement of volatile byproducts to analysis systems.
Core Takeaway Argon serves as both a protective shield and a delivery vehicle. It guarantees that the chemical breakdown is purely thermal (pyrolysis) rather than oxidative (burning), preserving the integrity of the solid biochar and ensuring accurate analysis of the evolved gases.

Creating a Controlled Reaction Environment
Eliminating the Risk of Combustion
The most immediate function of high-purity argon is to create an anaerobic (oxygen-free) zone within the reactor.
Solar pyrolysis involves extremely high temperatures. If air were allowed to enter the reaction zone, the biomass would ignite and burn rather than decompose. Argon effectively blocks this combustion pathway.
Ensuring Experimental Accuracy
For scientific validity, the degradation of the material must be driven solely by heat, not by chemical reactions with the atmosphere.
By maintaining an inert atmosphere, argon ensures that the resulting data reflects pure thermal degradation. This isolates the variables, allowing researchers to accurately attribute changes in the material to the solar energy input alone.
Facilitating Process Flow and Analysis
Acting as a Carrier Gas
Beyond protection, argon plays an active mechanical role as a carrier gas.
As the biomass breaks down, it releases various pyrolysis gases. The continuous flow of argon sweeps these gases away from the hot zone, preventing secondary reactions that could occur if the gases remained static in the reactor.
Enabling Steady-State Detection
The argon flow transports these generated gases to downstream cooling and detection systems.
Because the flow is continuous and steady, it allows for consistent, real-time monitoring of gas evolution. This steady transport is critical for quantitative analysis of the pyrolysis byproducts.
Protecting Material Integrity
Preserving Metallic States
In advanced pyrolysis applications involving metal-impregnated biomass, argon plays a critical chemical preservation role.
Specifically, if substances like copper nanoparticles are present, oxygen would degrade them into copper oxides. Argon protects these metals, maintaining them in their active, zero-valent state ($Cu^0$) which is essential for catalytic applications.
Maximizing Solid Yield
The presence of oxygen promotes the conversion of carbon into $CO_2$ and ash, reducing the amount of useful solid residue.
By excluding oxygen, the process favors the retention of carbon. This maximizes the yield of solid biochar, ensuring a higher quantity of the desired carbonaceous product.
Understanding the Trade-offs
Flow Rate Sensitivity
While continuous flow is necessary, the rate of flow must be calibrated carefully.
If the flow is too high, it may dilute the evolved gases, making detection difficult. If the flow is too low, residence time in the reactor increases, potentially altering the chemical composition of the oil and gas products through secondary cracking.
The Cost of Purity
Using high-purity argon is non-negotiable for avoiding trace oxidation, but it represents a significant operational cost.
Standard industrial argon may contain trace impurities that can skew sensitive experimental results or oxidize highly reactive nanoparticles. The investment in high purity is a requisite for data integrity, not just a luxury.
Making the Right Choice for Your Goal
Depending on the specific objectives of your pyrolysis project, your focus on the argon supply will shift:
- If your primary focus is Biochar Production: Prioritize the exclusion of oxygen to maximize carbon yield and prevent ash formation.
- If your primary focus is Gas Analysis: Focus on a precise, steady flow rate to ensure generated gases are transported to the detector without excessive dilution.
- If your primary focus is Catalyst Synthesis: Ensure extreme gas purity to prevent the oxidation of sensitive metal nanoparticles (like Copper) on the carbon substrate.
Ultimately, the continuous argon flow is the invisible backbone of the process, transforming a potential fire hazard into a precise, scientifically valid thermal reaction.
Summary Table:
| Function of Argon | Primary Benefit | Why It Matters |
|---|---|---|
| Inert Atmosphere | Prevents Combustion | Ensures thermal decomposition (pyrolysis) instead of burning. |
| Carrier Gas | Transport Mechanism | Moves volatile gases to detection systems for real-time analysis. |
| Chemical Shielding | Preserves Metal States | Protects nanoparticles (like Cu) from oxidation during the process. |
| Yield Optimization | Maximizes Biochar | Minimizes carbon loss to CO2, resulting in higher solid product yield. |
Achieve Scientific Precision in Solar Pyrolysis
Don't let trace oxidation or inconsistent gas flow compromise your research results. KINTEK provides advanced high-temperature lab solutions designed to maintain the rigorous inert environments your experiments demand.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your unique thermal processing needs. Whether you are optimizing biochar yield or synthesizing complex catalysts, our equipment ensures the thermal stability and atmospheric control required for success.
Ready to elevate your laboratory capabilities? Contact us today to discuss your custom furnace requirements.
Visual Guide
References
- Arturo Aspiazu-Méndez, Claudio A. Estrada. Analysis of the Solar Pyrolysis of a Walnut Shell: Insights into the Thermal Behavior of Biomaterials. DOI: 10.3390/en17061435
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role do high-precision laboratory ovens play in assessing the energy potential of MSW? Enhancing Biomass Accuracy
- What is the necessity of baking electrode sheets in a vacuum oven? Ensure Battery Stability and Peak Performance
- What is the significance of maintaining an inert nitrogen atmosphere during molten salt activation? Ensure Pore Purity
- How does a needle valve control silver foil surface quality for graphene growth? Prevent defects with pressure control.
- Why is electromagnetic induction heating considered environmentally friendly? Zero Emissions & High Efficiency
- Why is a laboratory vacuum oven required for GO slurry? Preserving Chemical Integrity in Graphene Oxide Dehydration
- What is the function of a Teflon-lined autoclave in hydrothermal acid treatment? Enhance Catalyst Synthesis Efficiency
- Why must High Vanadium High Speed Steel undergo multiple tempering cycles? Key to Unlocking Secondary Hardness



















