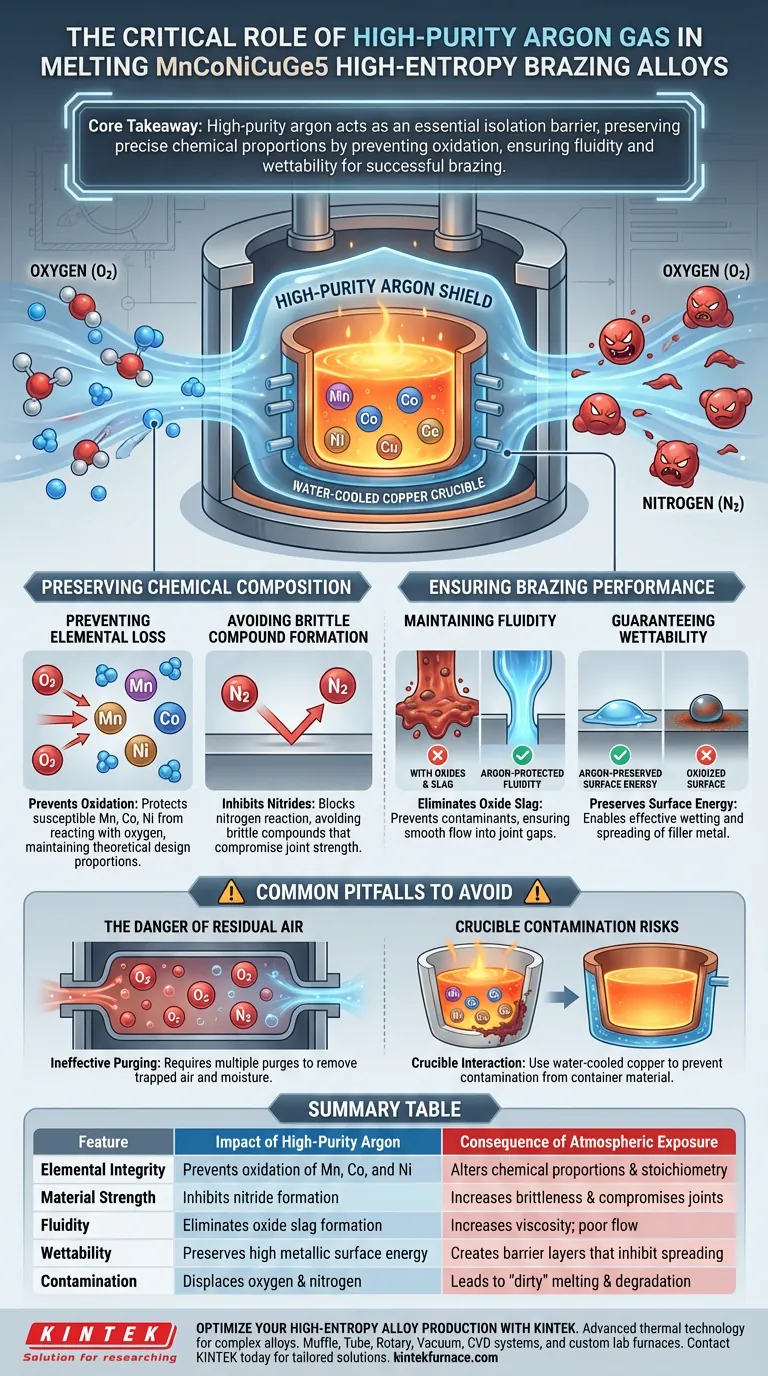
High-purity argon acts as an essential isolation barrier. During the melting of MnCoNiCuGe5 high-entropy brazing alloys, this inert gas serves to displace oxygen and nitrogen from the furnace environment. Its primary function is to prevent the oxidation of active elements—specifically manganese, cobalt, and nickel—which are highly susceptible to degradation at melting temperatures.
Core Takeaway The success of a high-entropy alloy depends entirely on maintaining precise chemical proportions. High-purity argon preserves this theoretical balance by preventing elemental loss through oxidation, directly ensuring the fluidity and wettability required for a successful braze.
Preserving Chemical Composition
The integrity of a high-entropy alloy lies in its complex chemical makeup. The melting stage is the most vulnerable point in the alloy's production cycle.
Preventing Elemental Loss
Active elements within the MnCoNiCuGe5 matrix, particularly manganese, cobalt, and nickel, react aggressively with oxygen. If exposed to air during melting, these elements will oxidize, effectively removing them from the metallic matrix. This loss alters the chemical proportions of the alloy, meaning the final product will no longer match the theoretical design.
Avoiding Brittle Compound Formation
Beyond simple oxidation, atmospheric nitrogen can also pose a threat at high temperatures. Without an argon shield, nitrogen can react with the melt to form nitrides. These compounds introduce brittleness to the alloy, severely compromising the mechanical strength of the final joint.
Ensuring Brazing Performance
For a brazing alloy to function, it must behave predictably in its liquid state. The atmosphere used during melting directly dictates this behavior.
Maintaining Fluidity
Oxides formed during melting act as contaminants that increase the viscosity of the molten metal. By maintaining a high-purity argon environment, you prevent the formation of oxide slag. This ensures the alloy remains fluid and flows freely into the joint gap during the brazing process.
Guaranteeing Wettability
Wettability is the ability of the liquid filler metal to spread across the base material. Oxidation creates a barrier layer that inhibits this spreading. The inert argon atmosphere preserves the metallic surface energy, ensuring the filler metal can wet the substrate effectively.
Common Pitfalls to Avoid
While using argon is standard, how you manage the environment is just as critical as the gas itself.
The Danger of Residual Air
Simply pumping argon into a furnace is often insufficient. The furnace chamber must be purged multiple times prior to melting to eliminate residual air and moisture trapped in the system. Failing to purge effectively will lead to "dirty" melting, even if argon is flowing during the heat cycle.
Crucible Contamination Risks
While argon protects the atmosphere, the containment vessel also plays a role in purity. Using a water-cooled copper crucible in conjunction with argon arc melting is recommended. This enables rapid cooling and prevents the crucible material itself from contaminating the sensitive high-entropy melt.
Making the Right Choice for Your Goal
To maximize the quality of your MnCoNiCuGe5 alloy, align your atmospheric control with your specific objectives.
- If your primary focus is Fundamental Research: Prioritize the purging cycle reliability to ensure the final stoichiometry matches your theoretical calculations exactly.
- If your primary focus is Brazing Application: Focus on maintaining a continuous inert overpressure to guarantee maximum fluidity and wettability in the final joint.
Strict control of the argon environment is not just a safety measure; it is the foundational step that dictates the metallurgical success of the alloy.
Summary Table:
| Feature | Impact of High-Purity Argon | Consequence of Atmospheric Exposure |
|---|---|---|
| Elemental Integrity | Prevents oxidation of Mn, Co, and Ni | Alters chemical proportions and stoichiometry |
| Material Strength | Inhibits nitride formation | Increases brittleness and compromises joints |
| Fluidity | Eliminates oxide slag formation | Increases viscosity; poor flow into joint gaps |
| Wettability | Preserves high metallic surface energy | Creates barrier layers that inhibit spreading |
| Contamination | Displaces oxygen and nitrogen | Leads to "dirty" melting and alloy degradation |
Optimize Your High-Entropy Alloy Production with KINTEK
Precision in brazing starts with an uncompromising melting environment. KINTEK provides the advanced thermal technology needed to maintain the integrity of complex alloys like MnCoNiCuGe5. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temp furnaces—all fully customizable to meet your unique atmosphere and purity requirements.
Don't let oxidation compromise your research or industrial applications. Contact KINTEK today to discover how our tailored furnace solutions can enhance your lab's efficiency and metallurgical success.
Visual Guide
References
- S.V. Maksymova, V.V. Voronov. Structure formation of seams using high-entropic brazing filler metal MnCoNiCuGe5. DOI: 10.21203/rs.3.rs-7260180/v1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What materials are commonly used for the crucible in an induction melting furnace? Choose the Right Crucible for Your Metal
- Why is multiple vacuum remelting necessary for Ti-33Mo-0.2C? Solve High-Moly Segregation Challenges
- What is the disadvantage of an induction furnace? Its Core Limitation in Metal Refining
- What role does a vacuum induction melting furnace play in CoCrFeMnNi production? Ensure Purity and Homogeneity
- How is heat generated in induction heating? Discover Efficient Non-Contact Heating Methods
- Why is induction heating faster than traditional methods? Achieve Instantaneous, Internal Heat Generation
- Where is induction heating commonly used? Discover Its Key Industrial and Commercial Applications
- In which industries is vacuum melting technology commonly applied? Essential for Aerospace, Medical, and Electronics
















