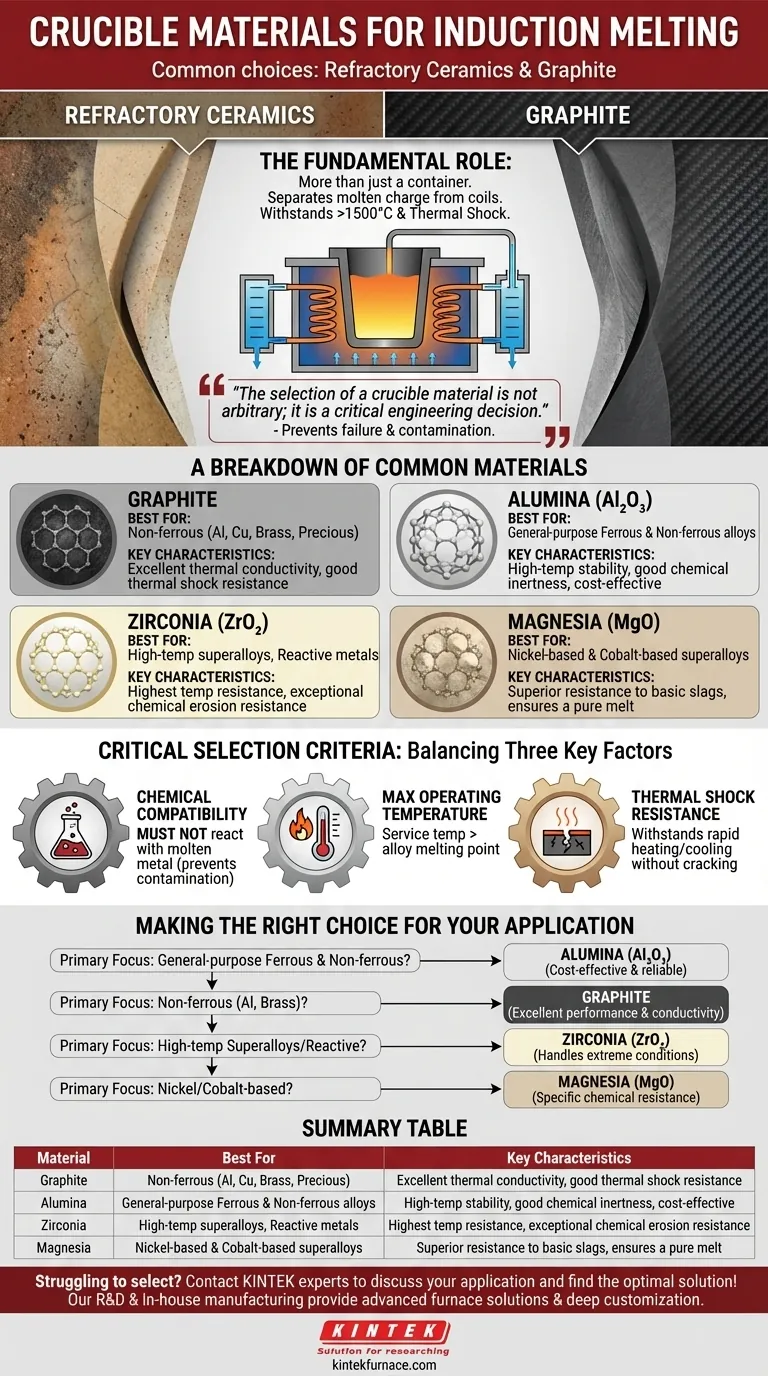
The most common materials for induction furnace crucibles are refractory ceramics and graphite. These materials are specifically chosen for their ability to withstand extreme temperatures without melting or reacting with the molten metal they contain.
The selection of a crucible material is not arbitrary; it is a critical engineering decision. The right choice is dictated by the chemical properties of the metal being melted and the maximum operating temperature, as a mismatch can lead to crucible failure and contamination of the final product.

The Fundamental Role of the Crucible
The crucible is the heart of the melting operation. It acts as the primary container, separating the molten metal charge from the water-cooled induction coils that generate the heat.
More Than Just a Container
Think of the crucible as a highly specialized, non-reactive vessel. Its primary job is to hold the metal charge securely as it transitions from a solid to a liquid state, often at temperatures exceeding 1500°C (2732°F).
Surviving Thermal Extremes
The crucible must endure immense thermal shock—the stress created by rapid temperature changes. It heats up incredibly fast during the melting cycle and cools relatively quickly afterward, a process that can easily crack inferior materials.
A Breakdown of Common Crucible Materials
While the broad categories are "ceramic" and "graphite," the specific material choice depends entirely on the application.
Graphite Crucibles
Graphite is an excellent conductor of heat, which can aid in efficient melting. It offers good thermal shock resistance and is often used for melting non-ferrous metals like aluminum, copper, and precious metals.
Alumina (Al₂O₃) Crucibles
Alumina is a versatile, widely used ceramic refractory. It provides high-temperature stability and good chemical inertness, making it a reliable choice for melting a wide range of both ferrous (iron and steel) and non-ferrous alloys.
Zirconia (ZrO₂) Crucibles
When temperatures are extremely high or the metal being melted is highly reactive, zirconia is a premium choice. It has a higher melting point than alumina and exhibits exceptional resistance to chemical erosion, making it suitable for specialty alloys and superalloys.
Magnesia (MgO) Crucibles
Magnesia crucibles are the material of choice for melting nickel-based and cobalt-based superalloys. They show superior resistance to the basic slags often generated during the processing of these advanced metals.
Understanding the Trade-offs: Critical Selection Criteria
Choosing the wrong crucible is a costly mistake that can ruin a batch of metal or damage the furnace itself. The decision hinges on balancing three key factors.
Chemical Compatibility
This is the most important consideration. The crucible material must not react with the molten metal. For example, melting a highly reactive titanium alloy in the wrong ceramic could cause the crucible to degrade and introduce oxygen impurities into the melt, compromising the final product's integrity.
Maximum Operating Temperature
The material must have a service temperature well above the melting point of the alloy. Zirconia is used for the most demanding high-temperature applications, while alumina is a robust workhorse for many standard ferrous and non-ferrous metals.
Thermal Shock Resistance
The ability to withstand rapid heating and cooling without cracking is crucial for furnace uptime and safety. Graphite and certain specially formulated ceramics are engineered to have excellent thermal shock resistance.
Making the Right Choice for Your Application
Your specific melting goal dictates the correct material. Always consult the manufacturer's specifications for your alloy and furnace.
- If your primary focus is general-purpose ferrous or non-ferrous alloys: An alumina (Al₂O₃) crucible is often the most cost-effective and reliable starting point.
- If your primary focus is non-ferrous metals like aluminum or brass: A graphite or clay-graphite crucible provides excellent performance and thermal conductivity.
- If your primary focus is high-temperature superalloys or reactive metals: A zirconia (ZrO₂) crucible is necessary to handle the extreme conditions and prevent contamination.
- If your primary focus is nickel or cobalt-based alloys: A magnesia (MgO) crucible offers the specific chemical resistance required to ensure a pure melt.
Selecting the correct crucible material is fundamental to ensuring the safety, efficiency, and quality of your melting operations.
Summary Table:
| Material | Best For | Key Characteristics |
|---|---|---|
| Graphite | Non-ferrous metals (Al, Cu, brass, precious) | Excellent thermal conductivity, good thermal shock resistance |
| Alumina (Al₂O₃) | General-purpose ferrous & non-ferrous alloys | High-temperature stability, good chemical inertness, cost-effective |
| Zirconia (ZrO₂) | High-temperature superalloys, reactive metals | Highest temperature resistance, exceptional chemical erosion resistance |
| Magnesia (MgO) | Nickel-based & cobalt-based superalloys | Superior resistance to basic slags, ensures a pure melt |
Struggling to select the perfect crucible material for your specific alloy and process?
At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, and Vacuum & Atmosphere Furnaces, is complemented by strong deep customization capabilities. We can help you select or custom-engineer a crucible and furnace system that precisely meets your unique experimental and production requirements, ensuring safety, efficiency, and the highest quality melt.
Contact our experts today to discuss your application and find the optimal solution!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What metals cannot be heated by induction? Understanding Material Suitability for Efficient Heating



















