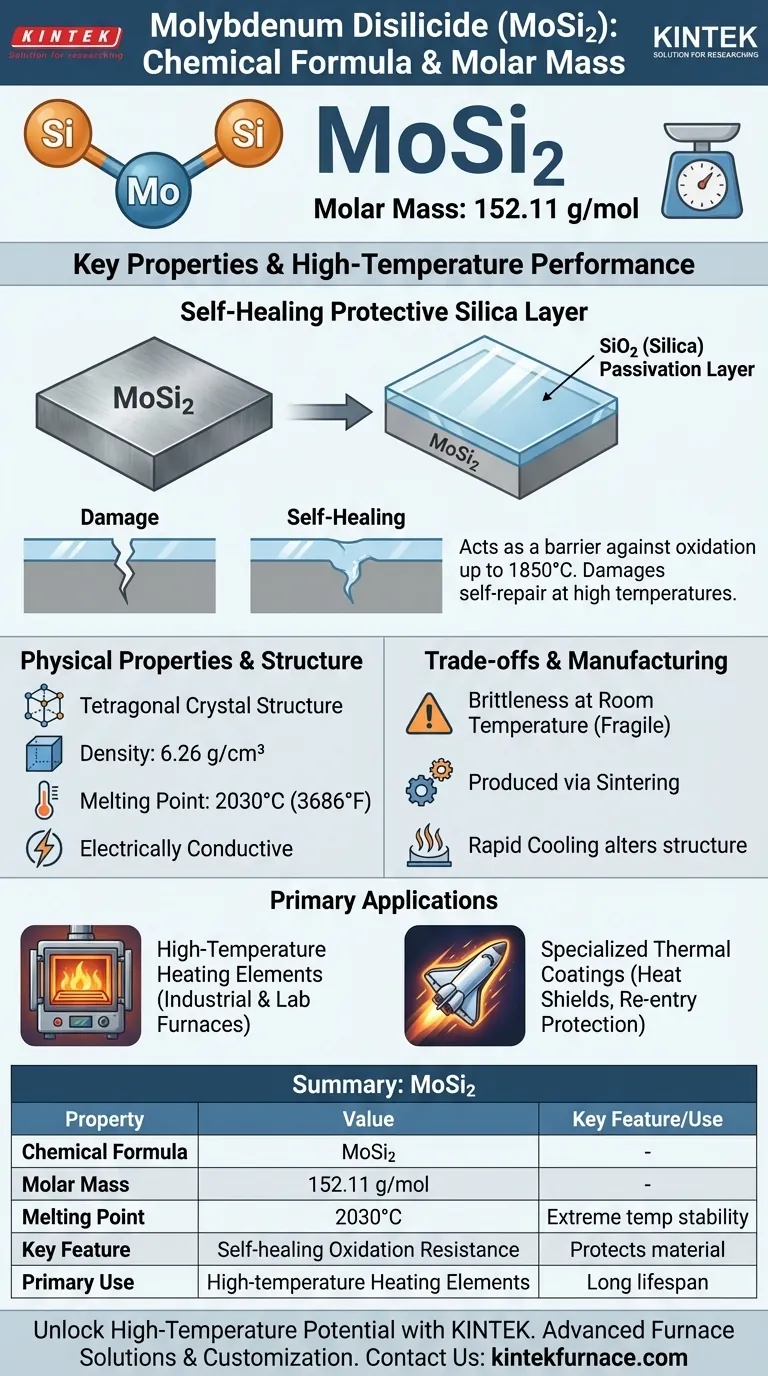
The chemical formula for molybdenum disilicide is MoSi₂. Its corresponding molar mass is 152.11 g/mol. This intermetallic compound is composed of one atom of molybdenum (Mo) for every two atoms of silicon (Si), giving it unique properties that make it a critical material in high-temperature applications.
While its chemical formula defines its composition, molybdenum disilicide's true value lies in its ability to form a protective, self-healing surface layer at extreme temperatures, making it one of the most durable materials for electric heating elements.

What is Molybdenum Disilicide?
Molybdenum disilicide is not simply a mixture; it is a specific intermetallic compound, often described as a cermet (ceramic-metal composite). This structure gives it a blend of metallic and ceramic-like properties.
Chemical Composition and Structure
MoSi₂ is a gray, metallic-looking solid. It possesses a tetragonal crystal structure, which is a key factor in determining its physical characteristics.
Key Physical Properties
The material is defined by its performance under extreme conditions. It has a moderate density of 6.26 g/cm³, a very high melting point of 2030°C (3686°F), and is electrically conductive, which allows it to function as a resistive heating element.
The Secret to Its High-Temperature Performance
The primary reason MoSi₂ is used in demanding environments is not just its high melting point, but its remarkable resistance to oxidation.
The Protective Silica Layer
When heated to high temperatures in an oxygen-rich atmosphere, the silicon in MoSi₂ reacts with oxygen to form a thin, non-porous passivation layer of pure silicon dioxide (SiO₂), which is essentially glass.
Why This "Passivation Layer" Matters
This SiO₂ layer acts as a robust barrier, protecting the underlying MoSi₂ from further oxidation and degradation. If the layer is damaged, the exposed material will simply form a new protective layer, making it self-healing. This property allows MoSi₂ elements to operate reliably at temperatures up to 1850°C.
Understanding the Trade-offs
No material is perfect. The exceptional high-temperature performance of MoSi₂ comes with a significant limitation at lower temperatures.
Brittleness at Room Temperature
Like many ceramics, molybdenum disilicide is very brittle and fragile when it is cold. It must be handled with care during installation and maintenance to avoid fractures. Its toughness only increases at high temperatures.
Manufacturing Considerations
MoSi₂ components are typically produced through sintering, a process of compacting and forming a solid mass of material by heat and pressure. Other methods like plasma spraying can be used, but rapid cooling may result in different crystalline forms (like β-MoSi₂) that can alter its properties.
Primary Applications Driven by Its Properties
The unique combination of electrical conductivity and extreme oxidation resistance defines the primary uses for MoSi₂.
High-Temperature Heating Elements
This is the most common application. MoSi₂ heating elements are valued for their long lifespan, stable electrical resistance, and ability to withstand rapid heating and cooling cycles without damage. This makes them ideal for industrial and laboratory furnaces.
Specialized Thermal Coatings
Due to its high emissivity (the ability to radiate thermal energy), MoSi₂ is also used as a coating for heat shields in highly specialized applications, such as protecting components during atmospheric re-entry.
Making the Right Choice for Your Application
Understanding the core properties of molybdenum disilicide allows you to decide if it is the correct material for your specific goal.
- If your primary focus is extreme temperature stability (above 1600°C) in an oxidizing atmosphere: MoSi₂ heating elements are the definitive choice due to their self-healing protective layer.
- If your primary focus is mechanical toughness at low temperatures: You must account for the material's inherent brittleness through careful system design and handling protocols.
- If your project requires rapid thermal cycling: The stable resistance and durability of MoSi₂ make it a superior choice over many other heating element materials.
By leveraging its unique ability to protect itself, you can achieve stable and reliable performance in the most demanding thermal environments.
Summary Table:
| Property | Value |
|---|---|
| Chemical Formula | MoSi₂ |
| Molar Mass | 152.11 g/mol |
| Melting Point | 2030°C (3686°F) |
| Key Feature | Self-healing oxidation resistance |
| Primary Use | High-temperature heating elements |
Unlock the potential of molybdenum disilicide in your lab with KINTEK's advanced high-temperature furnace solutions. Our Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, backed by deep customization, ensure precise performance for your unique experimental needs. Contact us today to enhance your high-temperature applications!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What makes SIC heating elements superior for high-temperature applications? Unlock Efficiency and Durability
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- Why are SIC heating elements resistant to chemical corrosion? Discover the Self-Protecting Mechanism
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights



















