The primary advantage of using nitrogen during alkaline silica extraction is its ability to create a chemically inert environment that strictly preserves the solution's pH levels. By displacing reactive atmospheric gases, nitrogen ensures the alkaline agent remains potent enough to fully dissolve silica from the biomass.
Nitrogen acts as a crucial process stabilizer, preventing atmospheric carbon dioxide from neutralizing the alkaline solvent. This protection ensures the chemical reaction focuses entirely on converting silicon into soluble sodium silicate, rather than being wasted on side reactions.

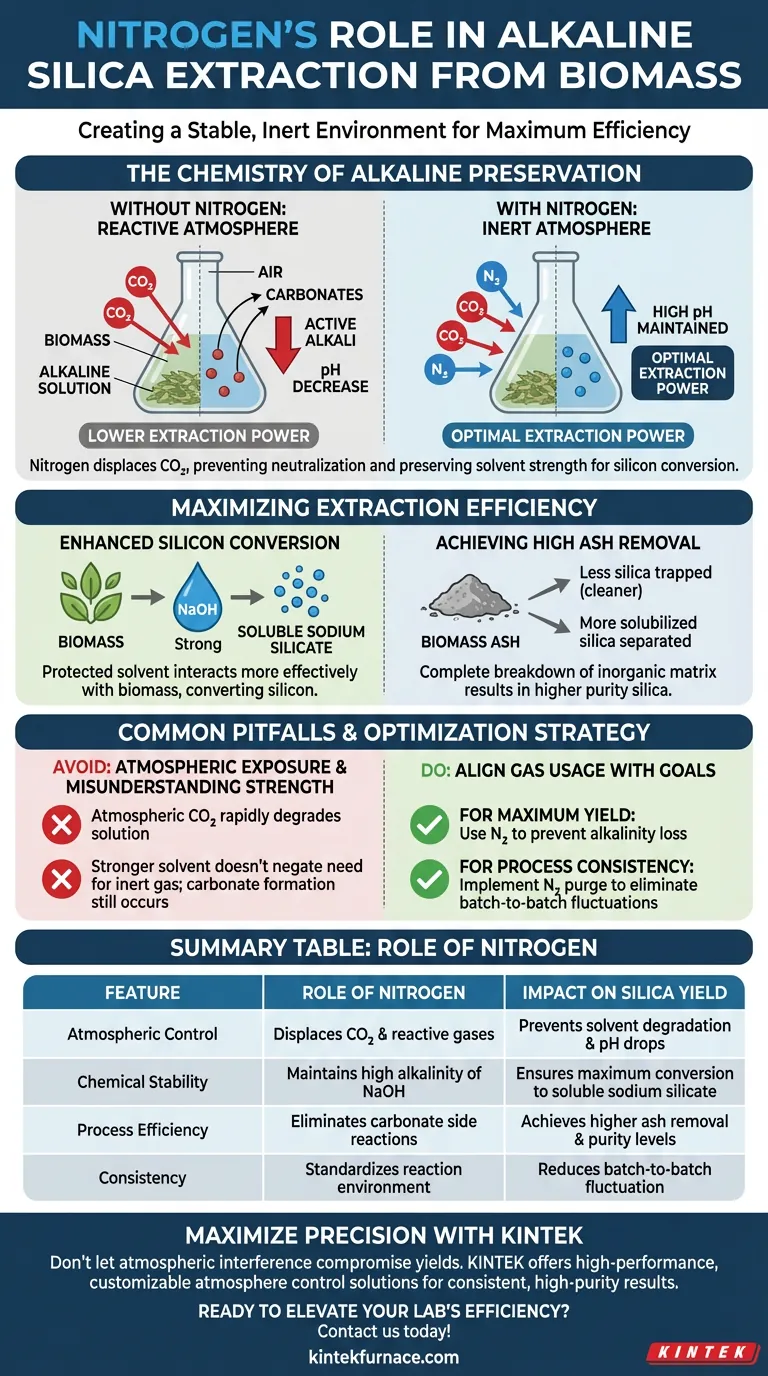
The Chemistry of Alkaline Preservation
Maintaining a High pH Environment
The extraction of silica from biomass relies heavily on maintaining a high pH within the alkaline solution.
Nitrogen is fundamentally non-reactive in this context. It does not dissolve into the solution to alter its acidity or basicity, ensuring the chemical environment remains stable throughout the process.
Preventing Carbonate Formation
The most significant threat to alkaline extraction is the presence of carbon dioxide ($CO_2$).
If the solution is exposed to air, $CO_2$ reacts with the alkaline agents (such as sodium hydroxide) to form carbonates. This reaction effectively consumes the active alkali, lowering the pH and reducing the solution's extraction power.
Displacing Reactive Gases
Nitrogen serves as a "filling gas" to physically displace these reactive elements.
By occupying the headspace or bubbling through the reactor, it prevents $CO_2$ from coming into contact with the solvent.
Maximizing Extraction Efficiency
Enhancing Silicon Conversion
The goal of the process is to convert inorganic silicon found in biomass into soluble sodium silicate.
This conversion efficiency is directly tied to the concentration and strength of the sodium hydroxide ($NaOH$). Because nitrogen protects the $NaOH$ from neutralization, the solvent can interact more effectively with the biomass.
Achieving High Ash Removal
When the solvent performs optimally, it breaks down the inorganic matrix of the biomass more completely.
This results in high ash removal rates, as the silica is successfully solubilized and separated from the organic material. Without nitrogen, the reduced alkalinity would leave significant amounts of silica trapped in the biomass ash.
Common Pitfalls to Avoid
The Cost of Atmospheric Exposure
Failing to use an inert gas like nitrogen is a common source of process inconsistency.
Operators often underestimate how quickly atmospheric $CO_2$ can degrade an alkaline solution. This degradation leads to unpredictable yields and requires higher concentrations of solvent to compensate for the loss.
Misunderstanding Solvent Strength
It is a mistake to assume that starting with a stronger solvent negates the need for an inert atmosphere.
Even highly concentrated solutions will suffer from surface carbonate formation without nitrogen, leading to lower purity in the final silica product.
Optimizing Your Extraction Strategy
To achieve the best results in silica extraction, align your gas usage with your specific processing goals:
- If your primary focus is Maximum Yield: Use nitrogen to prevent alkalinity loss, ensuring every mole of solvent is available to convert silicon.
- If your primary focus is Process Consistency: Implement a nitrogen purge to eliminate variable atmospheric conditions that cause batch-to-batch pH fluctuations.
Control over your reaction atmosphere is just as critical as the concentration of your chemical agents.
Summary Table:
| Feature | Role of Nitrogen in Extraction | Impact on Silica Yield |
|---|---|---|
| Atmospheric Control | Displaces $CO_2$ and reactive gases | Prevents solvent degradation and pH drops |
| Chemical Stability | Maintains high alkalinity of $NaOH$ | Ensures maximum conversion to soluble sodium silicate |
| Process Efficiency | Eliminates carbonate formation side reactions | Achieves higher ash removal and purity levels |
| Consistency | Standardizes reaction environment | Reduces batch-to-batch fluctuation in extraction rates |
Maximize Your Extraction Precision with KINTEK
Don’t let atmospheric interference compromise your silica yields. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable for your unique lab and industrial needs. Whether you are optimizing biomass processing or developing advanced materials, our high-temperature furnace solutions provide the precise atmosphere control you need for consistent, high-purity results.
Ready to elevate your lab's efficiency? Contact us today to consult with our experts on the perfect system for your extraction strategy!
Visual Guide
References
- Multi-step pre-treatment of rice husk for fractionation of components including silica. DOI: 10.3389/fchem.2025.1538797
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What critical protective roles does argon serve for AA7150-Al2O3 composites? Ensure Purity & Density
- What critical function does a high-temperature atmosphere sintering furnace serve? Engineer Advanced Nuclear Fuels
- Why is the choice of furnace atmosphere dependent on the specific process and material? Ensure Optimal Heat Treatment Results
- What are the design configurations of retort furnaces? Optimize Your Thermal Processing with the Right Setup
- What role does a high-temperature atmosphere furnace play in Ce3+ doped LCMS ceramics? Unlock Peak Luminescence
- What processes can be performed using a retort furnace? Unlock Precision Heat Treatment for Superior Materials
- What is the purpose of switching between N2 and H2 in electrical steel annealing? Master Atmosphere Control
- Why are retort furnaces significant in industrial applications? Unlock Precision Heat Treatment and Superior Material Quality



















