In short, the choice of furnace atmosphere is critical because it directly controls the chemical reactions that occur on your material's surface at high temperatures. The gas inside a furnace is not passive; it is an active ingredient in the heat-treating process. Selecting the wrong atmosphere can lead to undesirable outcomes like oxidation, embrittlement, or surface decarburization, effectively ruining the component, while the correct atmosphere is essential to achieving the desired material properties.
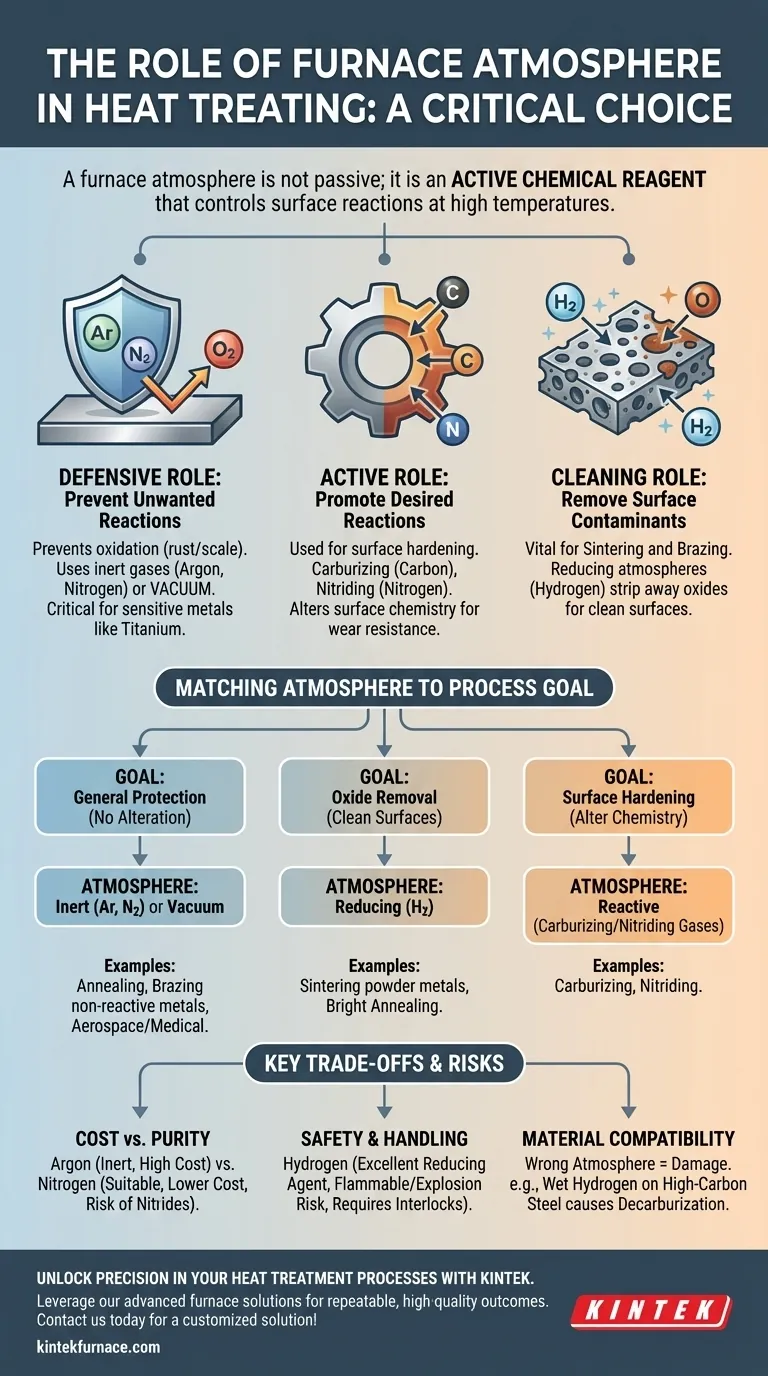
A furnace atmosphere is not merely a background gas; it is an active chemical reagent. The fundamental task is to select an atmosphere that either remains chemically neutral to your material or actively promotes a desired surface reaction while preventing destructive ones.

The Core Function of a Furnace Atmosphere
At its core, a controlled atmosphere allows you to dictate the chemical environment at elevated temperatures, where materials are most reactive. This control is exercised for two primary reasons: to defend the material or to actively change it.
The Defensive Role: Preventing Unwanted Reactions
The most common use of a furnace atmosphere is to protect the material from air, specifically oxygen. At high temperatures, most metals will readily oxidize (rust or scale) when exposed to oxygen.
An inert atmosphere, such as one filled with Argon or Nitrogen, acts as a protective blanket. It displaces the oxygen, preventing these unwanted reactions and ensuring the material's surface remains clean and unaltered.
A vacuum is the ultimate defensive atmosphere, removing virtually all gas molecules to create an environment where unwanted reactions are minimized. This is critical for highly reactive metals like titanium.
The Active Role: Promoting Desired Reactions
In more advanced processes, the atmosphere is used to intentionally change the surface of a material. The gas is chosen specifically to donate elements to the workpiece.
For example, in carburizing, a carbon-rich atmosphere is used to diffuse carbon atoms into the surface of steel, creating a hard, wear-resistant outer case.
Similarly, in nitriding, a nitrogen-rich atmosphere (often from dissociated ammonia) is used to form hard nitrides on the surface of steel components, increasing surface hardness and fatigue resistance.
The Cleaning Role: Removing Surface Contaminants
Some atmospheres are chosen for their ability to clean the material's surface. This is vital in processes like sintering and brazing, where clean surfaces are necessary for strong metallurgical bonds.
A reducing atmosphere, typically containing Hydrogen, is exceptionally effective at this. The hydrogen actively reacts with and strips away oxides from the surfaces of metal powders or parent metals, creating a chemically clean surface that is ready for bonding.
Matching the Atmosphere to the Material and Process
The specific combination of material and process goal dictates the optimal atmosphere. There is no single "best" choice; there is only the right choice for the application.
For General Protection: Inert Atmospheres or Vacuum
When the goal is simply to heat a material without altering it, an inert gas is the standard choice. This is common for annealing stainless steel to relieve stress or for brazing copper components.
A vacuum is used for the most sensitive materials or when even the slightest contamination is unacceptable, such as in aerospace or medical applications.
For Oxide Removal: Reducing Atmospheres
Processes like the sintering of metal powders rely on a reducing atmosphere. Without it, the individual powder grains would remain coated in an oxide layer, preventing them from fusing into a dense, solid part. Bright annealing of steel or copper also uses a reducing atmosphere to produce a clean, bright finish.
For Surface Hardening: Reactive Atmospheres
As mentioned, processes like carburizing and nitriding are entirely dependent on a reactive atmosphere to supply the necessary elements (carbon and nitrogen) for surface modification. The composition of this gas is precisely controlled to achieve a specific case depth and hardness.
Understanding the Trade-offs and Risks
Choosing an atmosphere involves balancing efficacy, cost, and safety. The ideal chemical choice may not always be the most practical one.
Cost vs. Purity
Argon is extremely inert but is significantly more expensive than Nitrogen. While Nitrogen is suitable for many applications, it can react with certain metals at high temperatures (like titanium and some stainless steels) to form undesirable nitrides.
Safety and Handling
Hydrogen is an outstanding reducing agent but is highly flammable and poses an explosion risk. Furnaces using hydrogen require specialized safety interlocks, ventilation, and handling procedures, which adds to operational complexity and cost.
Material Compatibility Failures
Using the wrong atmosphere can actively damage your material. A common mistake is using a "wet" hydrogen atmosphere (containing water vapor) when heat-treating high-carbon steel. This can cause decarburization, where carbon is stripped from the steel's surface, leaving it soft and unable to be hardened properly.
Making the Right Choice for Your Process
Your decision should be guided by a clear understanding of your material and your final objective.
- If your primary focus is preventing oxidation on non-reactive metals: A Nitrogen atmosphere often provides the best balance of cost and performance.
- If your primary focus is joining parts or working with sensitive materials: An Argon atmosphere or a vacuum is required to ensure the highest purity and prevent any reaction.
- If your primary focus is cleaning oxides for sintering or bright annealing: A reducing atmosphere containing Hydrogen is necessary to actively strip away surface oxides.
- If your primary focus is altering the material's surface chemistry: You must use a reactive atmosphere specifically designed for that process, such as a carburizing or nitriding gas mixture.
By treating the furnace atmosphere as a critical process variable, you gain precise control over your material's final properties and ensure repeatable, high-quality results.
Summary Table:
| Atmosphere Type | Primary Function | Common Applications |
|---|---|---|
| Inert (e.g., Argon, Nitrogen) | Prevents oxidation and contamination | Annealing, brazing of non-reactive metals |
| Vacuum | Minimizes all gas reactions | Aerospace, medical applications with sensitive materials |
| Reducing (e.g., Hydrogen) | Removes surface oxides | Sintering, bright annealing |
| Reactive (e.g., carburizing, nitriding gases) | Alters surface chemistry | Carburizing, nitriding for surface hardening |
Unlock Precision in Your Heat Treatment Processes with KINTEK
Struggling with material oxidation, inconsistent results, or surface defects in your high-temperature applications? KINTEK has the solution. Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with advanced high-temperature furnace solutions. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements.
Whether you need inert atmospheres for protection, reducing gases for cleaning, or reactive environments for surface hardening, our expertise ensures you achieve repeatable, high-quality outcomes. Don't let atmosphere choice compromise your process—contact us today to discuss how we can tailor a furnace system to your specific material and process goals.
Contact us now for a customized solution!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What is the relationship between temperature and the furnace atmosphere in material processing? Master the Critical Heat-Environment Balance
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What are the two main types of atmosphere furnaces and their characteristics? Choose the Right Furnace for Your Lab



















