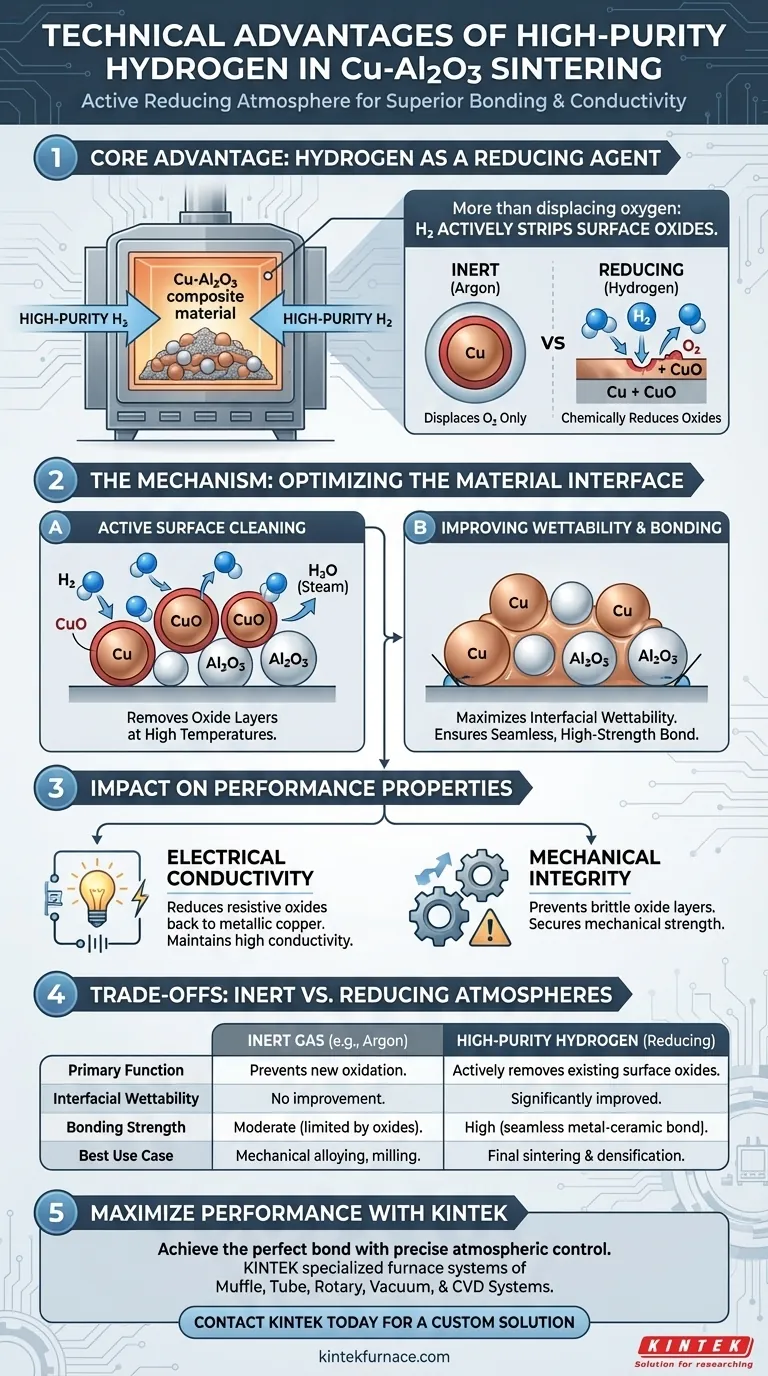
High-purity hydrogen serves as a critical reducing agent that actively improves the sintering process of Copper-Alumina (Cu-Al2O3) composites. Unlike inert atmospheres that simply displace oxygen, hydrogen chemically reacts with the material at high temperatures to strip away surface oxides. This active purification is the fundamental driver for achieving superior bonding strength and maintaining the high electrical conductivity required for advanced applications.
The core advantage of a hydrogen atmosphere is its ability to transform the material interface: by reducing surface oxides, it maximizes wettability, ensuring the copper matrix forms a seamless, high-strength bond with the alumina reinforcement.

The Mechanism of Oxide Reduction
Active Surface Cleaning
During the sintering process, copper particles are susceptible to surface oxidation. High-purity hydrogen acts as a strong reducing atmosphere, effectively removing these oxide layers from the particle surfaces as temperatures rise.
Prevention of Oxidation
Sintering occurs at high heat, where materials are most vulnerable to reacting with oxygen. Hydrogen creates a protective environment that prevents new oxidation from occurring, preserving the chemical purity of the copper matrix throughout the thermal cycle.
Optimizing the Material Interface
Improving Wettability
The presence of oxides creates a barrier that prevents molten or softening metals from adhering to other particles. By eliminating these oxides, hydrogen significantly improves the interfacial wettability between the copper matrix and the alumina (Al2O3) particles.
Enhancing Bonding Strength
Strong composites rely on the load transfer between the matrix and the reinforcement. The improved wettability fostered by hydrogen leads to tighter, more cohesive contact points, directly enhancing the interfacial bonding strength of the final composite.
Impact on Performance Properties
Maintaining Electrical Conductivity
Copper oxides are poor conductors and act as electrical resistance points within the material. By reducing these oxides back to metallic copper, the hydrogen atmosphere ensures the composite maintains high electrical conductivity.
Securing Mechanical Integrity
Weak interfaces lead to material failure under stress. The reduction of oxides ensures that the mechanical performance of the composite is not compromised by brittle oxide layers or poor particle adhesion.
Understanding the Trade-offs: Inert vs. Reducing Atmospheres
The Limitation of Inert Gases
It is important to distinguish between preventing oxidation and reversing it. While inert gases like Argon are excellent for preventing oxidation during mechanical alloying (milling) by isolating fresh surfaces, they cannot remove oxides that have already formed.
The Necessity of Reduction
If a powder has been exposed to even trace amounts of oxygen prior to sintering, an inert atmosphere will lock those oxides inside the final product. Hydrogen is technically superior for sintering because it actively corrects surface impurities, whereas inert gases only preserve the current state of the material.
Making the Right Choice for Your Goal
To maximize the performance of your Cu-Al2O3 composite, align your atmospheric choice with your specific processing stage:
- If your primary focus is mechanical alloying/milling: Use high-purity Argon to isolate fresh surfaces and prevent initial oxidation during high-energy grinding.
- If your primary focus is sintering/densification: Use high-purity Hydrogen to actively reduce existing surface oxides and maximize interfacial bonding.
By utilizing hydrogen during the sintering phase, you ensure that the inherent conductivity of copper and the strength of alumina are fully realized in the final composite.
Summary Table:
| Feature | High-Purity Hydrogen (Reducing) | Inert Gas (Argon/Nitrogen) |
|---|---|---|
| Primary Function | Actively removes surface oxides | Displaces oxygen to prevent new oxidation |
| Interfacial Wettability | Significantly improved | No improvement to existing surfaces |
| Bonding Strength | High (seamless metal-ceramic bond) | Moderate (limited by residual oxides) |
| Electrical Conductivity | Optimized by reducing resistive oxides | Limited by trapped oxide layers |
| Best Use Case | Final sintering and densification | Mechanical alloying and powder milling |
Maximize Your Material Performance with KINTEK
Achieving the perfect bond in Cu-Al2O3 composites requires precise atmospheric control and high-temperature reliability. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temperature furnaces—all fully customizable to meet your unique sintering needs.
Don't let surface oxides compromise your conductivity or mechanical integrity. Let our technical experts help you select the ideal furnace setup to optimize your reducing atmosphere processes.
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- Tawfik M. Ahmed. Development and characterization of Cu-Al2O3 metal matrix composites through powder metallurgy techniques. DOI: 10.33545/26646536.2025.v7.i2a.137
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does an industrial-grade atmospheric furnace play in fire simulation tests? Master ASTM Safety Standards
- Why must a high-purity argon protective atmosphere be maintained during mechanical alloying? Ensure Peak Material Purity
- Why is a water-cooling spray system implemented in annealing? Maximize Production Throughput & Material Quality
- What is an exothermic atmosphere in furnace applications? Protect Metals from Oxidation Efficiently
- Why is a cylindrical atmosphere furnace utilized for the salt removal process in porous stainless steel fabrication?
- How does a high-temperature furnace facilitate flash pyrolysis? Unlock Superior Fe-N-C Catalyst Performance
- How are retort furnaces utilized in laboratory environments? Unlock Precise Atmospheric Control for Advanced Research
- How does the experimental box type atmosphere furnace ensure accurate atmosphere control? Master Precise Gas Management for Reliable Results



















