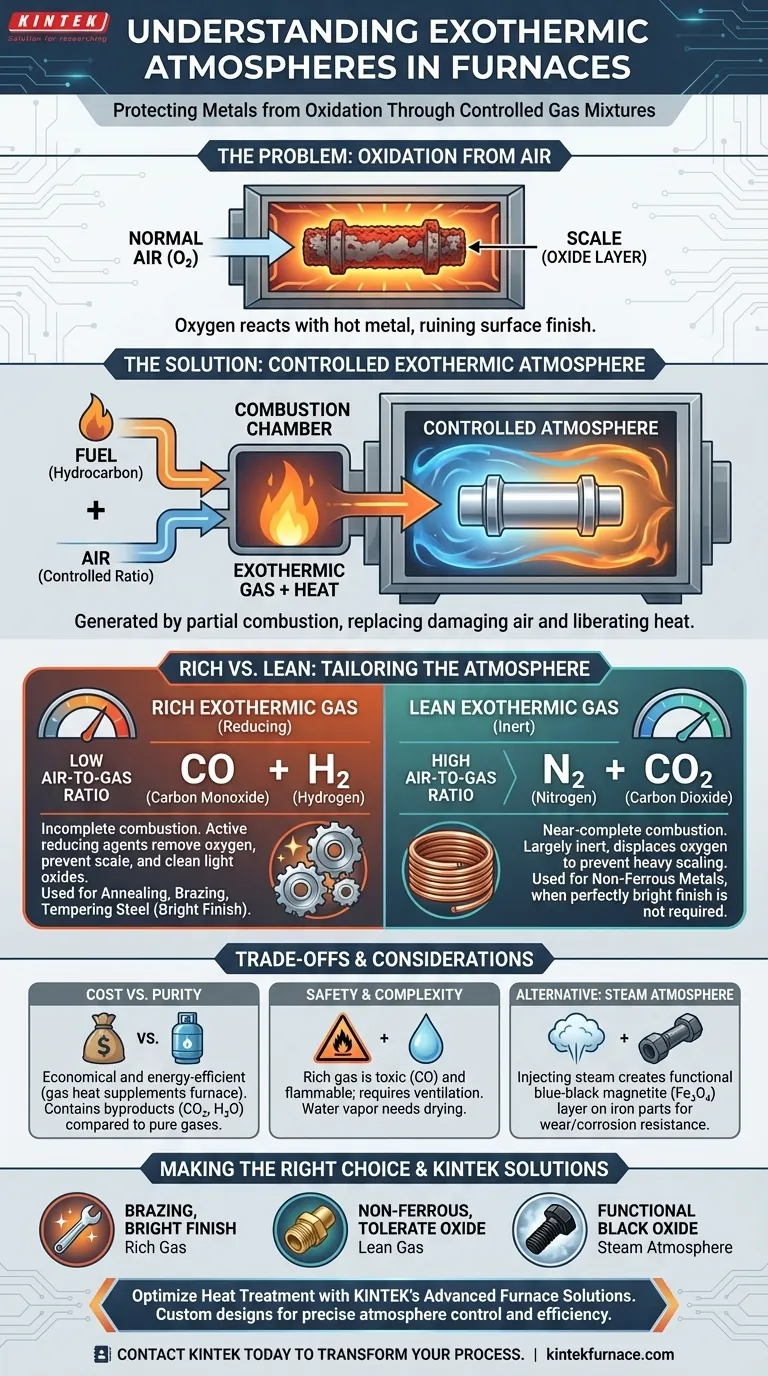
In furnace applications, an exothermic atmosphere is a precisely controlled gas mixture generated inside or near a furnace to protect metal parts from oxidation during heat treatment. This protective blanket is created through the partial combustion of a hydrocarbon fuel and air, a chemical reaction that liberates its own heat—hence the name "exothermic." This process replaces the damaging oxygen-rich air with a gas tailored to the specific metal and process.
The core purpose of an exothermic atmosphere is not just to heat a part, but to control the chemical environment at high temperatures. It transforms the furnace from a simple oven into a reactive chamber that can prevent scaling, preserve surface finish, and ensure the final metallurgical properties of the component.

The Fundamental Problem: Oxidation
Why Normal Air is the Enemy of Hot Metal
When you heat most metals, especially steel, in the presence of oxygen from the air, a chemical reaction occurs. This reaction forms an oxide layer on the surface, commonly known as scale.
This scale is detrimental. It ruins the surface finish, can interfere with subsequent processing like plating or painting, and represents a loss of material.
The Solution: A Controlled Atmosphere
To prevent this, heat treaters replace the air inside the furnace with a controlled atmosphere. An exothermic atmosphere is one of the most common and cost-effective types.
It's created by burning a fuel, typically natural gas, with a limited and controlled supply of air. The resulting flue gas is then conditioned (often cooled and dried) and piped into the furnace.
Rich vs. Lean: Tailoring the Atmosphere
The properties of an exothermic atmosphere are determined by the air-to-gas ratio used during its generation. This leads to two distinct categories: rich and lean.
Rich Exothermic Gas
A rich exothermic atmosphere is created using a low air-to-gas ratio, resulting in incomplete combustion. This produces a gas rich in carbon monoxide (CO) and hydrogen (H₂).
These two gases are powerful reducing agents. This means they will actively react with and remove oxygen, not only preventing scale from forming but also capable of cleaning light oxides already on a part's surface. It is used for annealing, brazing, and tempering steel.
Lean Exothermic Gas
A lean exothermic atmosphere is created with an air-to-gas ratio that is much closer to enabling complete combustion. The resulting gas is composed primarily of nitrogen (N₂) and **carbon dioxide (CO₂) **, with very little or no CO and H₂.
This atmosphere is largely inert and non-reactive. While not actively reducing like a rich gas, it effectively displaces oxygen to prevent heavy scaling. It is used when a perfectly bright finish is not required or when a very thin, controlled oxide layer is acceptable or even desired.
Understanding the Trade-offs
Cost-Effectiveness vs. Gas Purity
Exothermic atmospheres are often the most economical choice. The fuel used to generate the gas also produces heat, which can supplement the furnace's heating system, improving overall energy efficiency.
However, the resulting gas contains byproducts like carbon dioxide and water vapor, which can be undesirable for highly sensitive materials. Atmospheres generated from dissociated ammonia or pure cryogenic nitrogen and hydrogen are much purer, but also significantly more expensive.
Safety and Operational Complexity
The presence of high concentrations of carbon monoxide in rich exothermic gas makes it toxic and flammable. Proper ventilation, monitoring, and safety protocols are absolutely critical when working with it.
Furthermore, the water vapor produced during combustion can be oxidizing to steel at certain temperatures. For many processes, the gas must pass through a refrigerator or desiccant dryer to lower its dew point before it can be used.
Comparing to Alternatives: Steam Atmospheres
Another option for certain applications is a steam atmosphere. This is not an exothermic gas but serves a similar protective purpose.
Injecting steam into a furnace for tempering or stress-relieving iron-based parts creates a specific, uniform blue-black magnetite (Fe₃O₄) oxide layer. Unlike destructive red rust or scale, this layer improves wear and corrosion resistance.
Making the Right Choice for Your Process
Choosing the correct atmosphere is critical for a successful heat treatment outcome.
- If your primary focus is brazing, annealing, or achieving a bright finish on carbon steels: A rich exothermic atmosphere is an effective and economical choice.
- If your primary focus is treating non-ferrous metals or you can tolerate a slight, uniform oxide: A lean exothermic atmosphere provides sufficient protection at a lower cost and with fewer safety concerns.
- If your primary focus is creating a functional, corrosion-resistant black oxide finish on iron parts: A steam atmosphere is the specialized tool for that specific goal.
Ultimately, selecting the right furnace atmosphere is a deliberate engineering decision that directly impacts the quality, performance, and cost of your finished part.
Summary Table:
| Aspect | Details |
|---|---|
| Definition | Controlled gas mixture from partial combustion of hydrocarbon fuel and air, releasing heat to protect metals during heat treatment. |
| Types | Rich (high CO/H₂ for reducing oxides) and Lean (high N₂/CO₂ for inert protection). |
| Key Benefits | Prevents oxidation, controls surface finish, cost-effective, and energy-efficient. |
| Common Uses | Annealing, brazing, tempering of steels; suitable for non-ferrous metals with lean type. |
| Safety Notes | Rich type is toxic and flammable; requires ventilation and drying for moisture control. |
Optimize Your Heat Treatment with KINTEK's Advanced Furnace Solutions
Struggling with metal oxidation or inconsistent results in your lab processes? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide tailored high-temperature furnace solutions. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all designed with deep customization capabilities to meet your unique experimental needs—whether you're working with carbon steels, non-ferrous metals, or specialized alloys.
By choosing KINTEK, you'll benefit from precise atmosphere control, enhanced efficiency, and reliable performance, ensuring superior metallurgical properties and cost savings. Don't let oxidation compromise your outcomes—contact us today to discuss how our expertise can transform your heat treatment processes and deliver the results you deserve!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments



















