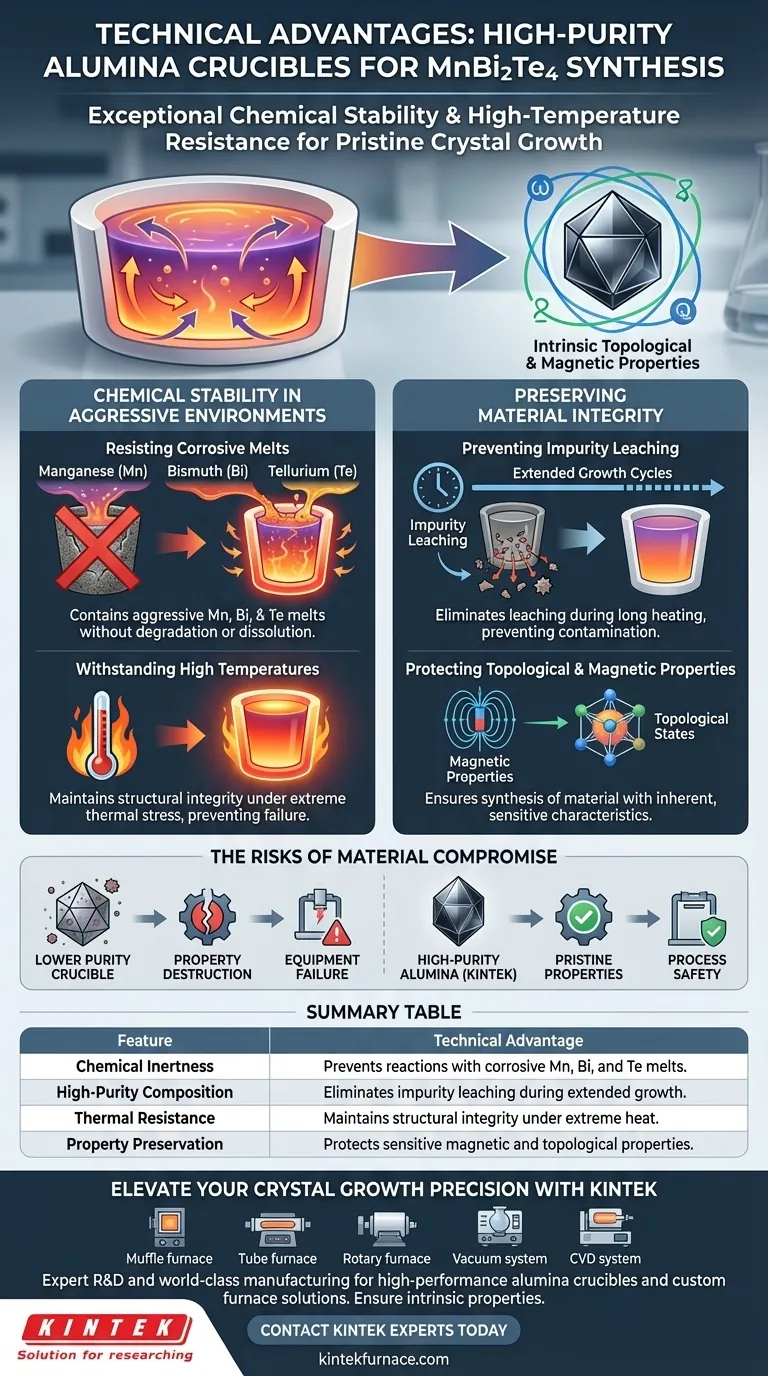
The primary technical advantages of using a high-purity alumina crucible for the synthesis of MnBi2Te4 are its exceptional chemical stability and high-temperature resistance. Specifically, this material is required to withstand the corrosive nature of active metal melts—Manganese (Mn), Bismuth (Bi), and Tellurium (Te)—while strictly preventing the introduction of impurities that would degrade the crystal's sensitive physical properties.
Success in synthesizing MnBi2Te4 relies on maintaining absolute chemical purity during extended heating. High-purity alumina acts as a stable barrier, preventing reaction with active melts and ensuring the final crystal retains its intrinsic topological properties.

Chemical Stability in Aggressive Environments
Resisting Corrosive Melts
The synthesis of MnBi2Te4 involves working with active metal melts, specifically Manganese, Bismuth, and Tellurium.
These elements are highly corrosive in their molten state. High-purity alumina provides the necessary inertness to contain these aggressive fluids without degrading or dissolving into the melt.
Withstanding High Temperatures
The growth process requires elevated temperatures to ensure the materials melt and react correctly.
High-purity alumina possesses exceptional high-temperature resistance, allowing it to maintain structural integrity throughout the heating process. This ensures the crucible does not fail or deform under the thermal stress required for synthesis.
Preserving Material Integrity
Preventing Impurity Leaching
One of the most critical aspects of MnBi2Te4 synthesis is the duration of the process, often involving extended growth cycles.
During these long periods of heating, a standard crucible might release contaminants. The high-purity characteristics of alumina prevent the leaching of unwanted impurities into the crystal lattice.
Protecting Topological and Magnetic Properties
The utility of MnBi2Te4 lies in its specific magnetic and topological properties.
These properties are extremely sensitive to defects and foreign atoms. By preventing impurity introduction, high-purity alumina ensures the synthesized material maintains the inherent characteristics required for advanced physics applications.
The Risks of Material Compromise
The Consequence of Impurities
It is vital to understand that the choice of crucible is not merely about containment, but about chemical isolation.
Using a crucible with lower purity levels or inferior chemical stability introduces a high risk of contaminating the melt. Even trace impurities can disrupt the crystal structure, effectively destroying the material's value for topological research.
Durability During Synthesis
The corrosive nature of Mn, Bi, and Te melts attacks weaker materials rapidly.
Failure to use a crucible capable of withstanding this specific chemical environment can lead to equipment failure during the growth cycle, resulting in the loss of the entire batch.
Making the Right Choice for Your Goal
Selecting the correct crucible is a foundational step in successful material synthesis.
- If your primary focus is Crystal Quality: Prioritize high-purity alumina to prevent contaminant leaching that destroys magnetic and topological states.
- If your primary focus is Process Safety: Rely on high-purity alumina to prevent containment failure caused by the corrosive interaction of Mn, Bi, and Te melts.
High-purity alumina provides the essential neutral environment required to transform reactive elements into a pristine topological insulator.
Summary Table:
| Feature | Technical Advantage in MnBi2Te4 Synthesis |
|---|---|
| Chemical Inertness | Prevents reactions with corrosive Manganese, Bismuth, and Tellurium melts. |
| High-Purity Composition | Eliminates impurity leaching during extended high-temperature growth cycles. |
| Thermal Resistance | Maintains structural integrity under extreme heat without deformation. |
| Property Preservation | Protects the sensitive magnetic and topological properties of the crystal. |
Elevate Your Crystal Growth Precision with KINTEK
Successful synthesis of complex materials like MnBi2Te4 demands equipment that never compromises on purity. KINTEK provides high-performance high-purity alumina crucibles and laboratory solutions designed to withstand the most aggressive chemical environments.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside customizable high-temp lab furnaces tailored to your unique research needs. Ensure your topological insulators maintain their intrinsic properties by choosing the right containment partner.
Ready to optimize your synthesis process? Contact our technical experts today to find the perfect solution for your laboratory.
Visual Guide
References
- Yaoxin Li, Chang Liu. Fabrication-induced even-odd discrepancy of magnetotransport in few-layer MnBi2Te4. DOI: 10.1038/s41467-024-47779-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- Why is the use of high-purity alumina crucibles essential for the synthesis of Ni3In2Se2? | Precision Material Purity
- What are the considerations for using vacuum-sealed quartz tubes for Ti-xCr-2Ge alloys? Ensure Peak Alloy Performance
- What functions do alumina crucibles and quartz tube encapsulation serve? Essential Shields for Na2In2As3 Synthesis
- What role do mass flow controllers play in gasification? Achieve Precise Atmosphere Control in Lab Furnaces
- What considerations lead to the selection of a corundum crucible for CVD sulfurization? Ensure Peak Sample Purity
- Why is a vacuum drying oven necessary for Al2O3/TiC ceramic powders? Ensure Purity and Prevent Agglomeration
- What is the technical necessity of using a glass boat in a pyrolysis furnace? Precision in Thermal Decomposition
- What is the function of a vacuum drying oven for biochar FTIR analysis? Ensure High-Purity Sample Preparation















