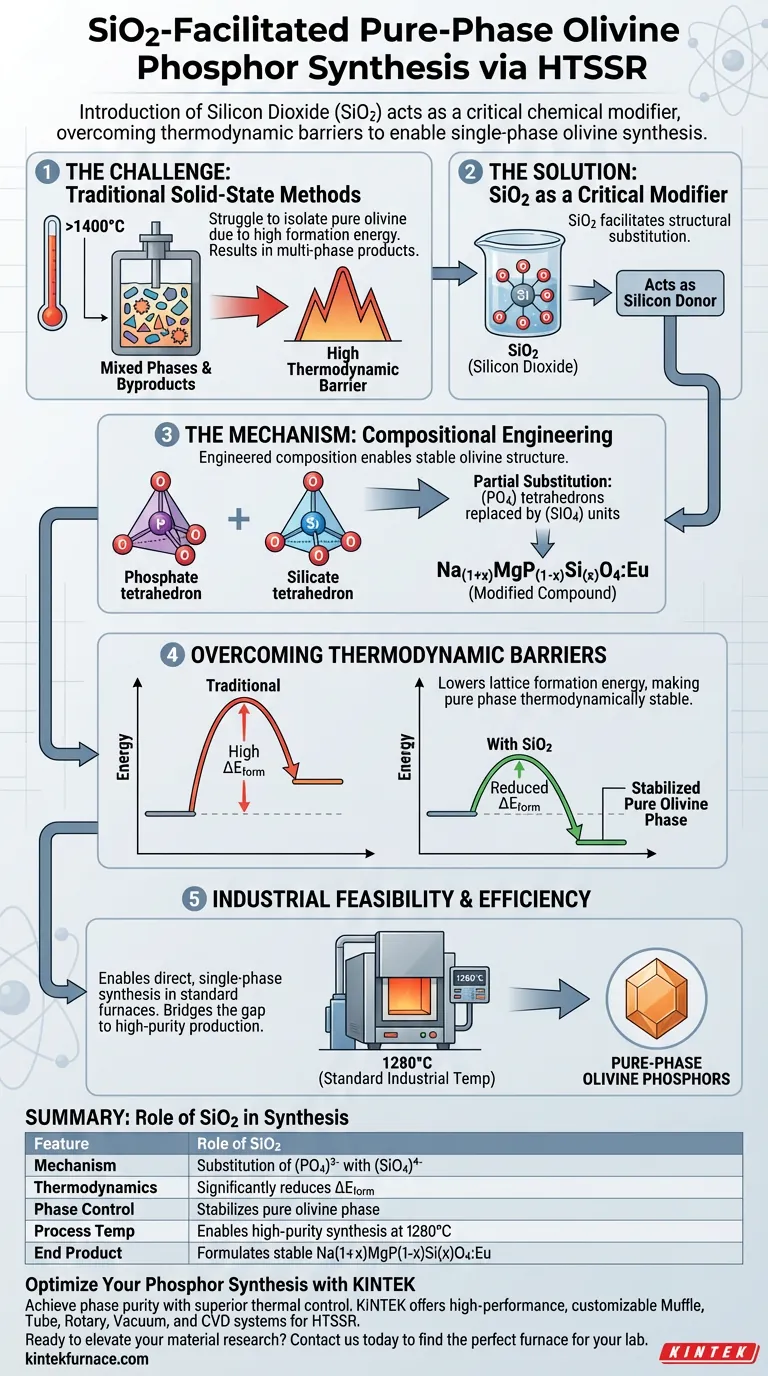
The introduction of silicon dioxide (SiO2) acts as a critical chemical modifier, enabling the synthesis of pure-phase olivine phosphors by altering the material's structural thermodynamics. By serving as a silicon source, SiO2 allows for the partial substitution of phosphate ($\text{PO}_4$) tetrahedrons with silicate ($\text{SiO}_4$) units. This substitution lowers the formation energy of the crystal lattice, making the thermodynamically stable pure olivine phase accessible at standard industrial temperatures ($1280^\circ\text{C}$).
Traditional solid-state methods struggle to isolate the pure olivine phase due to high thermodynamic barriers. Integrating SiO2 facilitates a specific structural substitution that significantly reduces formation energy, allowing for direct, single-phase synthesis in standard high-temperature furnaces.

The Mechanism of Compositional Engineering
Substituting Phosphate with Silicate
The primary function of silicon dioxide in this process is to act as a silicon donor. This enables a structural shift where silicate tetrahedrons ($\text{SiO}_4$) partially replace phosphate tetrahedrons ($\text{PO}_4$) within the crystal lattice.
Creating the Modified Compound
This substitution leads to the formation of a chemically modified compound with the formula $\text{Na}{1+x}\text{MgP}{1-x}\text{Si}_x\text{O}_4:\text{Eu}$. This specific composition is engineered to facilitate the stability of the olivine structure.
Overcoming Thermodynamic Barriers
Reducing Formation Energy
The most significant impact of SiO2 introduction is thermodynamic. The compositional change significantly reduces the formation energy ($\Delta E_{\text{form}}$) required to build the crystal lattice.
Stabilizing the Pure Phase
By lowering the energy threshold, the pure olivine phase becomes thermodynamically more stable. This stability is the key factor that allows the material to form as a single, cohesive phase rather than a mixture of unwanted byproducts.
Industrial Feasibility and Process Efficiency
Enabling High-Temperature Synthesis
Because the formation energy is lowered, the material can be synthesized effectively at $1280^\circ\text{C}$. This temperature range is perfectly compatible with industrial-grade high-temperature solid-state reaction (HTSSR) furnaces.
Solving the Purity Challenge
Historically, obtaining a single pure phase of olivine phosphors using traditional solid-state methods was difficult. The SiO2 modification strategy effectively bridges this gap, ensuring a pure product without the need for exotic processing conditions.
Understanding the Synthesis Context
The Limitation of Traditional Methods
It is important to recognize that without SiO2, the reaction lacks the necessary thermodynamic driver to settle into a pure phase. Traditional methods often fail to overcome the energy barriers required to isolate the single olivine structure.
The Role of Chemical Modification
This process is not merely about adding an ingredient; it is about chemical modification. The strategy relies on changing the fundamental composition of the material to engineer a path of least resistance for phase formation.
Implications for Material Synthesis
To achieve high-quality olivine phosphors, consider the following based on your specific objectives:
- If your primary focus is Phase Purity: Utilize SiO2 to facilitate the $\text{PO}_4$ to $\text{SiO}_4$ substitution, which is the chemical driver for isolating the single olivine phase.
- If your primary focus is Industrial Scaling: Leverage the reduced formation energy to perform synthesis at $1280^\circ\text{C}$, utilizing standard industrial HTSSR equipment rather than specialized laboratory setups.
By leveraging compositional engineering, you can transform a difficult multi-phase synthesis into a reliable, thermodynamically favored process.
Summary Table:
| Feature | Role of SiO2 in Synthesis |
|---|---|
| Mechanism | Substitution of (PO₄)³⁻ with (SiO₄)⁴⁻ tetrahedrons |
| Thermodynamics | Significantly reduces lattice formation energy (ΔE_form) |
| Phase Control | Stabilizes pure olivine phase; prevents unwanted byproducts |
| Process Temp | Enables high-purity synthesis at standard 1280°C (HTSSR) |
| End Product | Formulates stable Na1+xMgP1-xSixO4:Eu phosphors |
Optimize Your Phosphor Synthesis with KINTEK
Achieving phase purity in advanced material synthesis requires both precise chemical engineering and superior thermal control. KINTEK provides the high-performance heating solutions necessary to master the HTSSR process.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temperature furnaces are fully customizable to meet your unique thermodynamic requirements, ensuring consistent results for your olivine phosphor production.
Ready to elevate your material research? Contact us today to find the perfect furnace for your lab.
Visual Guide
References
- Jianwei Qiao, Lei Wang. Compositional engineering of phase-stable and highly efficient deep-red emitting phosphor for advanced plant lighting systems. DOI: 10.1038/s41377-024-01679-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the purpose of using a high-vacuum pump system for NiTi thin films? Ensure Pure Stoichiometry & Performance
- How does a vacuum drying oven contribute to stable lithium-selenium battery electrodes? Ensure Purity and Performance
- What is the purpose of performing homogenization at 1250°C? Optimizing Sintered Cobalt-Based Superalloys
- What are the technical advantages of using the molten salt method? Elevate Your Biomass Carbon Support Synthesis
- What are the advantages of using KOH as a chemical activator? Enhance Biomass Carbon Surface Area and Porosity
- What role does the addition of NaCl as a diluent play in the SHS of Titanium Diboride? Master Nano-Powder Synthesis
- What are the requirements for ovens in MOF synthesis? Achieve Precision Thermal Stability for High-Crystallinity
- Why is a furnace with high-precision temperature control required for DPKB-S? Ensuring Material Synthesis Accuracy



















