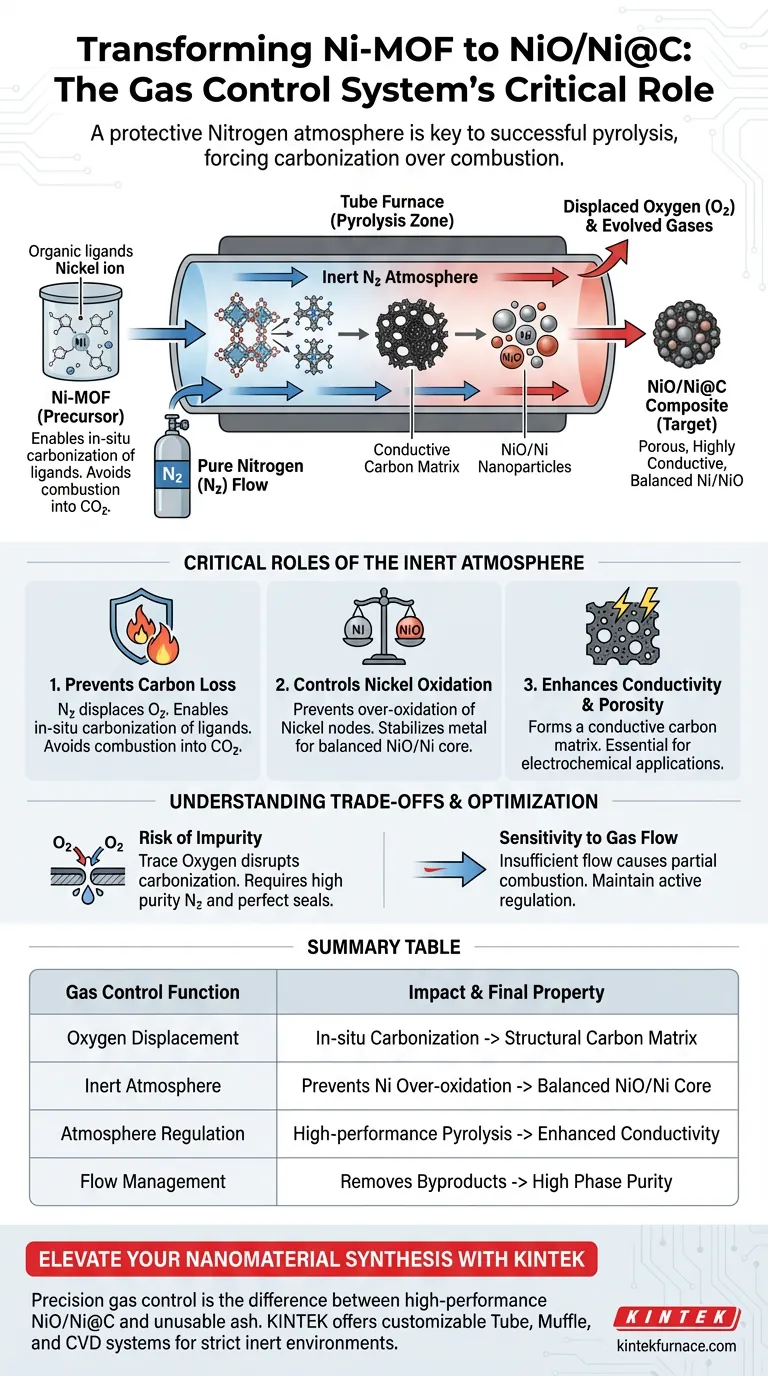
The gas control system is the primary mechanism for regulating the chemical environment within the tube furnace, specifically by establishing and maintaining a protective nitrogen atmosphere. This system ensures that the pyrolysis of the Nickel Metal-Organic Framework (Ni-MOF) occurs in strictly inert conditions, which is the defining factor in successfully synthesizing the target composite.
Core Takeaway: By displacing oxygen with nitrogen, the gas control system forces the organic ligands to carbonize rather than combust. This creates a conductive carbon matrix that stabilizes nickel nanoparticles, preventing their over-oxidation and resulting in the specific NiO/Ni@C composite structure.

The Critical Role of the Inert Atmosphere
Preventing Carbon Loss
The most immediate function of the nitrogen flow is to displace atmospheric oxygen.
Without this exclusion of oxygen, the high temperatures required for pyrolysis would cause the organic ligands in the MOF to combust.
Instead of burning away into carbon dioxide, the gas control system ensures these ligands undergo in-situ carbonization, preserving the carbon to form a structural matrix.
Controlling Nickel Oxidation States
The reference material highlights that the system specifically prevents the over-oxidation of nickel nanoparticles.
In an uncontrolled atmosphere, the nickel nodes within the MOF would likely oxidize completely, losing the desired metallic characteristics required for the Ni/NiO balance.
The inert nitrogen environment stabilizes the metal, allowing for the formation of the complex NiO/Ni core within the composite.
Enhancing Conductivity and Porosity
The successful carbonization of the ligands leads to the formation of a porous, highly conductive carbon-based composite.
This porosity is essential for the material's surface area, while conductivity is vital for its electrochemical applications.
The gas control system is the variable that determines whether you end up with high-performance conductive carbon or non-conductive ash.
Understanding the Trade-offs
The Risk of Impurity
While the system is designed to provide a protective atmosphere, its effectiveness relies entirely on the purity of the nitrogen source and the integrity of the seal.
Even trace amounts of oxygen leaking into the system can disrupt the carbonization process.
Sensitivity to Gas Flow
The "protection" offered by the gas system is not passive; it requires active regulation.
Insufficient flow may fail to flush evolved gases or incoming air, leading to partial combustion and a degradation of the carbon matrix.
Optimizing Your Synthesis Strategy
To ensure the successful transformation of Ni-MOF into NiO/Ni@C, focus on the following operational goals:
- If your primary focus is conductivity: Ensure the gas system maintains a slightly positive pressure to prevent any air ingress that could consume the carbon matrix.
- If your primary focus is specific stoichiometry (Ni vs. NiO): Verify the absolute purity of your nitrogen source to prevent uncontrolled oxidation of the nickel nanoparticles.
The gas control system is not merely a safety feature; it is the active chemical agent that dictates the phase purity and structural integrity of your final nanomaterial.
Summary Table:
| Gas Control Function | Impact on Transformation | Final Material Property |
|---|---|---|
| Oxygen Displacement | Enables in-situ carbonization of ligands | Structural carbon matrix |
| Inert Atmosphere | Prevents over-oxidation of Nickel | Balanced NiO/Ni core phases |
| Atmosphere Regulation | Facilitates high-performance pyrolysis | Enhanced conductivity & porosity |
| Flow Management | Removes evolved byproduct gases | High phase purity & integrity |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision gas control is the difference between high-performance NiO/Ni@C and unusable ash. Backed by expert R&D and manufacturing, KINTEK offers advanced Tube, Muffle, Rotary, Vacuum, and CVD systems designed to maintain the strict inert environments required for MOF transformation.
Our lab high-temperature furnaces are fully customizable to meet your specific pressure and purity needs, ensuring your carbonization processes deliver maximum conductivity and structural integrity. Don't leave your research to chance—contact our technical specialists today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Setayesh Darvishi, Kimia Zarean Mousaabadi. Design and fabrication of electrochemical sensor based on NiO/Ni@C-Fe3O4/CeO2 for the determination of niclosamide. DOI: 10.1038/s41598-024-58319-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is zonal heating in a horizontal tube furnace? Master Precise Thermal Control for Your Lab
- What are the applications of a laboratory tube furnace in chemical research? Unlock Precise High-Temperature Synthesis
- What is the function of a two-zone tube furnace in NiPS3 crystal growth? Master CVT for High-Quality Crystals
- What is the significance of expanding raw material applicability in tube furnaces? Unlock Versatility and Cost Savings
- What role does a horizontal quartz tube furnace play in the synthesis of Bi2Se3? Optimize CVD Nanosheet Production
- What is the function of a vacuum tube furnace in the regeneration of expanded graphite? Deep Pore Restoration Expert
- What advanced control features do modern tube furnaces have? Precision Temperature, Atmosphere, and Data Control
- Why is a specialized tube furnace with a steam inlet required for the steam activation of carbon materials?



















