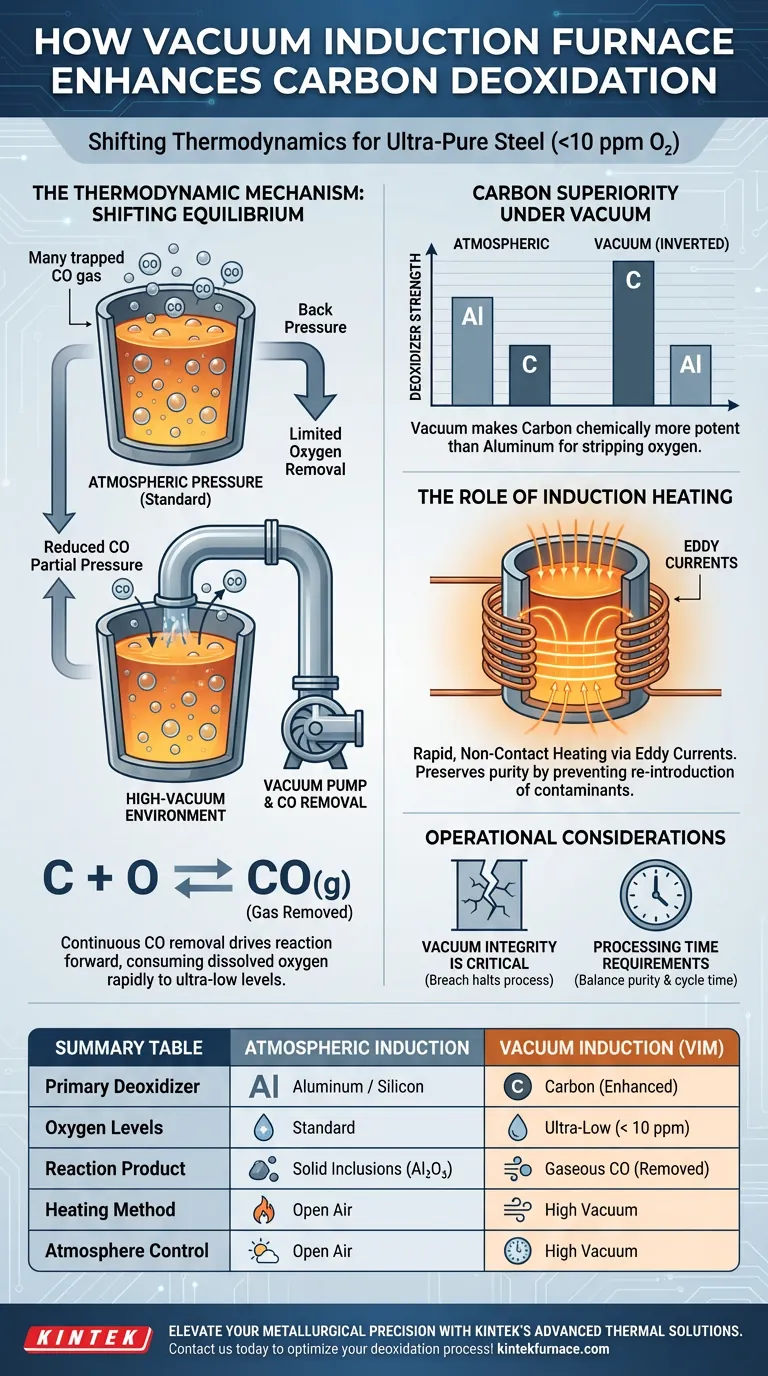
A vacuum induction furnace system enhances carbon deoxidation by actively reducing the partial pressure of carbon monoxide (CO) in the gas phase surrounding the melt. This physical change in the environment shifts the thermodynamic equilibrium, compelling carbon and dissolved oxygen to react more aggressively to form CO gas. This mechanism allows carbon to function as a superior deoxidizer compared to standard atmospheric processes.
By leveraging a high-vacuum environment, the system alters chemical thermodynamics to make carbon a stronger deoxidizer than aluminum, capable of reducing oxygen content in steel to ultra-pure levels below 10 ppm.
The Thermodynamic Mechanism
Shifting the Reaction Equilibrium
The fundamental reaction in this process involves carbon and oxygen combining to form carbon monoxide (CO) gas.
In atmospheric conditions, the surrounding pressure suppresses the escape of CO, which limits how much oxygen can be removed.
The vacuum system continuously evacuates the gas phase, effectively removing the "back pressure" on the reaction.
Driving Oxygen Depletion
By constantly removing the CO product, the system forces the chemical reaction to move forward to produce more gas.
This consumes the dissolved oxygen in the molten steel rapidly.
It continues until the oxygen concentration drops to extremely low levels, often achieving purities impossible in open-air furnaces.
Carbon Superiority Under Vacuum
Thermodynamically, aluminum is usually considered a stronger deoxidizer than carbon at standard pressures.
However, the vacuum environment inverts this relationship.
Because the reaction product (CO) is a gas that is removed by the vacuum, carbon becomes chemically more potent than aluminum for stripping oxygen from the melt.
The Role of Induction Heating
Generating Heat via Eddy Currents
While the vacuum controls the chemistry, the induction system provides the necessary energy.
Alternating current is passed through copper coils surrounding the crucible.
This generates eddy currents directly within the metal charge, creating intense heat from within.
Non-Contact Purity Preservation
The induction method allows for rapid heating without any physical contact between a fuel source and the metal.
This is critical for high-purity steel, as it prevents the re-introduction of contaminants during the heating phase.
It ensures that the low oxygen levels achieved by the vacuum are not compromised by the heating mechanism.
Operational Considerations and Trade-offs
Vacuum Integrity is Critical
The enhanced deoxidation capability is entirely dependent on maintaining a high-vacuum state.
Any breach or leak in the vacuum chamber will immediately increase the partial pressure of CO.
This would instantly halt the enhanced deoxidation process and potentially reverse the equilibrium.
Processing Time Requirements
While the thermodynamics favor deoxidation, the reaction is not instantaneous.
The process requires sufficient time for the carbon atoms to physically encounter oxygen atoms within the melt.
Operators must balance the need for extreme purity with the cycle time required for the reaction to reach equilibrium.
Making the Right Choice for Your Project
The combination of vacuum pressure and induction heating offers specific advantages depending on your metallurgical goals.
- If your primary focus is ultra-high purity: Rely on the vacuum phase to drive oxygen levels below 10 ppm, a threshold unreachable by atmospheric induction alone.
- If your primary focus is process cleanliness: Utilize the non-contact nature of induction heating to prevent contamination from fuel sources or electrodes.
By manipulating pressure to favor gas formation, you transform carbon from a standard alloying element into the most effective purification tool in your arsenal.
Summary Table:
| Feature | Atmospheric Induction | Vacuum Induction (VIM) |
|---|---|---|
| Primary Deoxidizer | Aluminum / Silicon | Carbon (Enhanced by Vacuum) |
| Oxygen Levels | Standard (Higher PPM) | Ultra-Low (< 10 ppm) |
| Reaction Product | Solid Inclusions (Al₂O₃) | Gaseous CO (Removed via Pump) |
| Heating Method | Non-contact Eddy Currents | Non-contact Eddy Currents |
| Atmosphere Control | Open Air or Inert Gas | High Vacuum (Reduced CO partial pressure) |
Elevate your metallurgical precision with KINTEK’s advanced thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers state-of-the-art Vacuum, CVD, Muffle, and Rotary systems designed to achieve ultra-pure results like sub-10 ppm oxygen levels. Whether you need a standard laboratory furnace or a fully customizable high-temperature system for specialized steelmaking, our experts are ready to engineer the perfect solution for your unique needs. Contact KINTEK today to optimize your deoxidation process!
Visual Guide
References
- Fang Gao, Yanping Bao. The Research on Carbon Deoxygenation of Molten Steel and Its Application in the Converter Steelmaking Process. DOI: 10.3390/met15060648
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- How does induction heating work in a vacuum environment? Achieve High-Purity, Contamination-Free Heat Treatment
- How do induction melting furnaces ensure superior metal quality? Achieve Purity, Homogeneity & Control
- What is the role of a vacuum arc furnace in the synthesis of AlCrFeNi HEAs? Achieve High-Purity Material Homogeneity
- Why are graphite crucibles and induction furnaces equipped with protective gas systems used for Zn-SiC composites?
- In what ways are induction furnaces cost-effective? Unlock Major Energy & Material Savings
- What materials and specifications are typically used in vacuum casting? Master High-Fidelity Prototyping with PU Resins
- What role does a VIM furnace play in Fe-32Mn-11Al-1.4C-3Ni steel? Precision Purity and Oxidation Protection
- What role does a Vacuum Induction Melting Furnace play in the production of weather-resistant steel? Precision Engineering



















