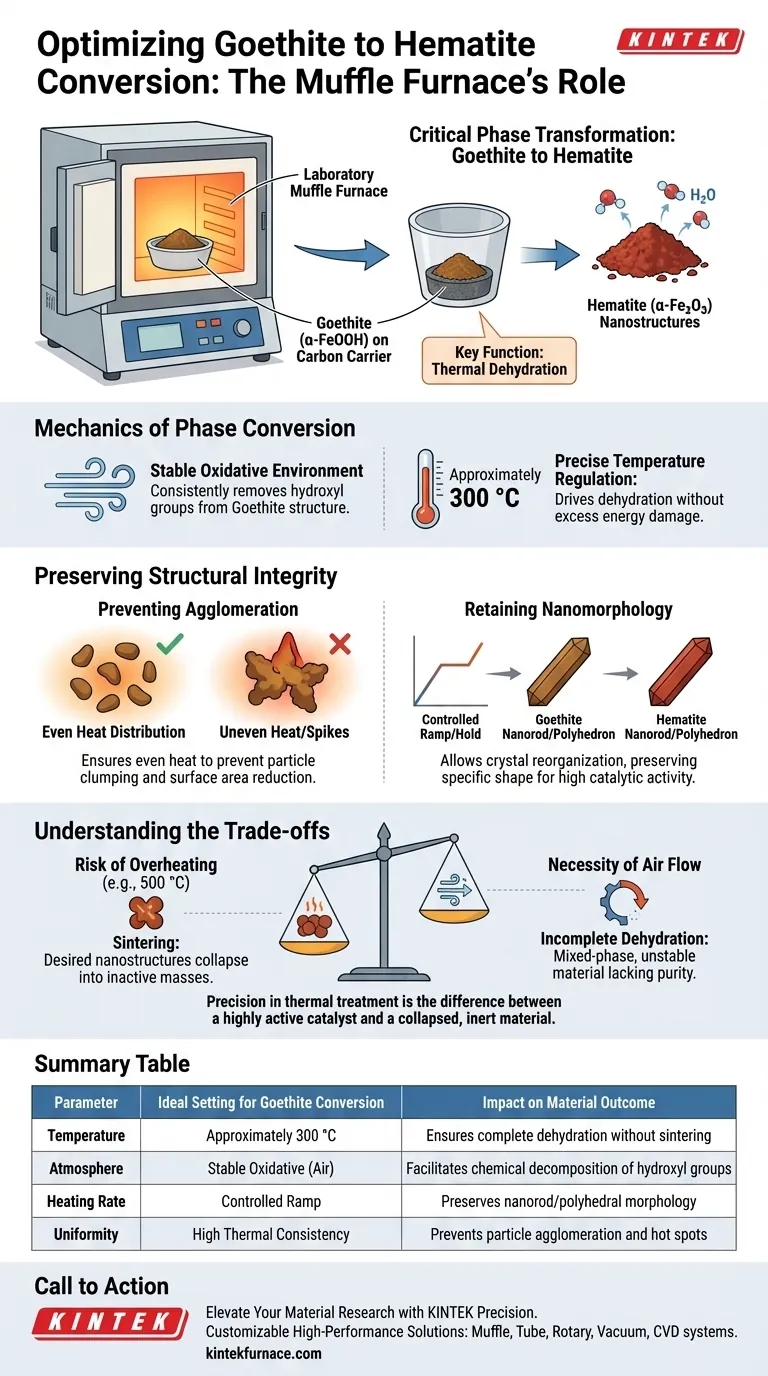
A laboratory muffle furnace drives the critical phase transformation of goethite into hematite through precise thermal dehydration. By maintaining a stable oxidative environment at approximately 300 °C, the furnace removes hydroxyl groups from the goethite (α-FeOOH) structure, effectively converting it into the more active hematite (α-Fe2O3) phase while loaded on carbon carriers.
The muffle furnace acts as a morphological stabilizer during the chemical conversion process. Its primary value lies not just in heating, but in controlling the rate of dehydration to ensure the resulting hematite retains the specific nanorod or polyhedral shape required for high catalytic activity.

The Mechanics of Phase Conversion
Thermal Dehydration
The core function of the furnace in this context is to facilitate the removal of water molecules chemically bound within the goethite structure.
This is not merely drying; it is a chemical decomposition where α-FeOOH releases water to become α-Fe2O3.
The muffle furnace provides the consistent air environment necessary for this oxidation reaction to occur uniformly across the sample.
Precise Temperature Regulation
For the specific conversion of goethite to hematite, the furnace must maintain a temperature of approximately 300 °C.
This specific thermal set point is critical because it provides enough energy to drive the dehydration reaction without supplying excess energy that could damage the material.
Preserving Structural Integrity
Preventing Agglomeration
One of the greatest risks during heat treatment is the clumping, or agglomeration, of particles.
If the heating is uneven or the temperature spikes uncontrollably, the active species on the carbon carrier will fuse together.
The laboratory muffle furnace mitigates this by ensuring an even heat distribution, preventing severe agglomeration that would reduce the surface area and effectiveness of the material.
Retaining Nanomorphology
The catalytic performance of the final product depends heavily on its shape, specifically its nanorod or polyhedral morphology.
The muffle furnace’s controlled ramp and hold times allow the crystal structure to reorganize from goethite to hematite without collapsing.
This preservation of shape ensures that the active sites remain accessible in the final hematite product.
Understanding the Trade-offs
The Risk of Overheating
While high temperatures are necessary for conversion, exceeding the optimal 300 °C range presents significant risks for this specific material.
Higher temperatures, such as the 500 °C range often used for industrial-grade metal salt decomposition or different supports (like Ti-Al), can be detrimental here.
Excessive heat can cause sintering, where the desired nanostructures collapse into larger, less active masses.
The Necessity of Air Flow
A muffle furnace typically operates with an air atmosphere, which is required for oxidation.
However, if the airflow is restricted or the environment is not sufficiently oxidative, the dehydration process may be incomplete.
This results in a mixed-phase material that lacks the purity and stability of fully converted hematite.
Making the Right Choice for Your Goal
To maximize the efficacy of your post-treatment process, align your furnace settings with your specific material requirements.
- If your primary focus is preserving nanostructure: Maintain the temperature strictly around 300 °C to ensure the goethite converts to hematite without losing its nanorod or polyhedral morphology.
- If your primary focus is preventing particle fusing: Prioritize a furnace with high thermal uniformity to avoid hot spots that cause severe agglomeration of the active species on the carbon carrier.
Precision in thermal treatment is the difference between a highly active catalyst and a collapsed, inert material.
Summary Table:
| Parameter | Ideal Setting for Goethite Conversion | Impact on Material Outcome |
|---|---|---|
| Temperature | Approximately 300 °C | Ensures complete dehydration without sintering |
| Atmosphere | Stable Oxidative (Air) | Facilitates chemical decomposition of hydroxyl groups |
| Heating Rate | Controlled Ramp | Preserves nanorod/polyhedral morphology |
| Uniformity | High Thermal Consistency | Prevents particle agglomeration and hot spots |
Elevate Your Material Research with KINTEK Precision
Don’t let unpredictable heat treatment compromise your catalytic results. KINTEK provides high-performance laboratory solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, engineered for the rigorous demands of thermal dehydration and phase conversion.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet your unique temperature uniformity and atmospheric needs, ensuring your nanostructures remain intact and active.
Ready to optimize your material transformation? Contact us today to find your custom furnace solution!
Visual Guide
References
- M. Antonia López-Antón, Ana Arenillas. Mercury Removal by Carbon Materials with Emphasis on the SO <sub>2</sub> –Porosity Relationship. DOI: 10.1002/open.202500190
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the function of a high-temperature lift furnace in the sintering process of SSZ electrolyte pellets?
- What features might a high-performance modern Muffle Furnace include? Discover Precision, Control, and Efficiency
- What is the critical role of a high-temperature muffle furnace in converting biomass into Fe-N-BC?
- How does a high-temperature box muffle furnace convert mussel shells to calcium oxide? Expert Calcination Guide
- Why is precise temperature control in a muffle furnace critical for 1250°C homogenization of AlCoCrFeNi alloys?
- What role do muffle furnaces play in sintering 3D-printed beta-TCP? Optimize Your Bioceramic Results
- What precautions should be taken with the thermostat before an experiment? Ensure Accuracy and Safety in Your Lab
- What is the temperature of a muffle oven? A Guide to Choosing the Right Range for Your Lab



















