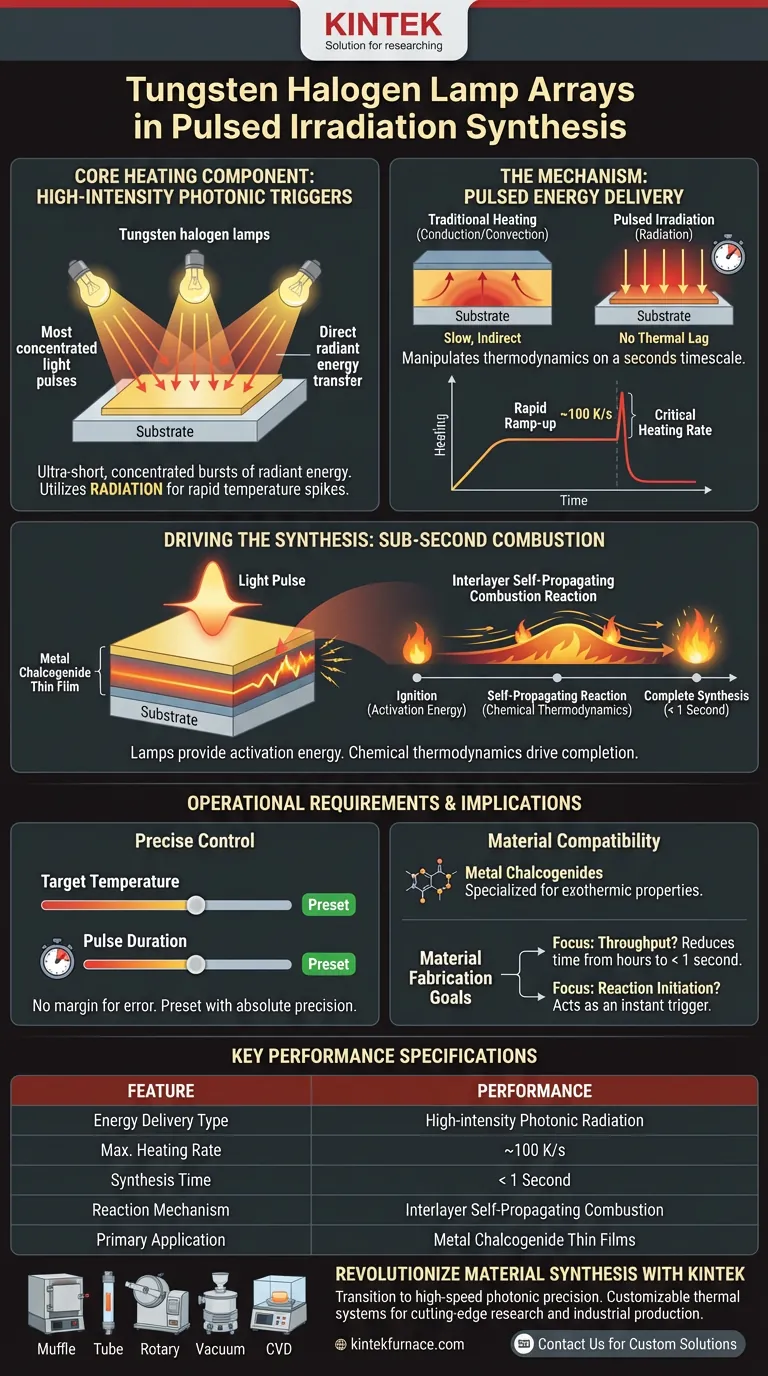
Tungsten halogen lamp arrays function as high-intensity photonic triggers that deliver ultra-short, concentrated bursts of radiant energy to thin film samples. Rather than heating materials slowly through conduction or convection, these arrays utilize radiation to achieve rapid temperature spikes, capable of heating rates as high as approximately 100 Kelvin per second (K/s).
The defining characteristic of this technology is speed: by delivering a high-energy pulse that creates instantaneous heat, these arrays trigger an interlayer self-propagating combustion reaction, allowing the complete chemical synthesis of metal chalcogenides in less than one second.

The Mechanism of Pulsed Energy Delivery
To understand why tungsten halogen lamps are the core component of this synthesis method, one must look at how they deliver energy compared to traditional thermal processing.
High-Intensity Radiation
The arrays operate as sources of high-intensity radiation. They do not rely on heating the surrounding air to warm the sample.
Instead, they project energy directly onto the thin film surface. This allows for immediate energy transfer with minimal thermal lag.
Ultra-Short Energy Pulses
The system is designed to provide energy in ultra-short pulses rather than a continuous steady state.
This pulsing capability allows the hardware to manipulate the thermodynamics of the sample on a timescale of seconds. It creates a specific thermal environment that traditional furnaces cannot replicate.
Driving the Synthesis Reaction
The primary goal of the tungsten halogen array is not just to "heat" the material, but to initiate a specific chemical chain reaction.
Achieving Critical Heating Rates
The arrays can drive heating rates of approximately 100 K/s. This rapid ramp-up is essential for bypassing lower-temperature equilibrium phases.
By bringing the sample to a preset temperature almost instantly, the system forces the material into a reactive state immediately.
Triggering Self-Propagating Combustion
The heat provided by the lamps serves as the ignition for an interlayer self-propagating combustion reaction.
Once the lamps raise the material to the ignition temperature, the reaction propagates through the layers of the film on its own. The lamps provide the activation energy, but the chemical thermodynamics drive the completion.
Sub-Second Synthesis
Because of this combustion mechanism, the actual synthesis does not require prolonged baking.
The entire chemical conversion of metal chalcogenides is completed in less than one second. This makes the tungsten halogen array a critical enabler for ultra-fast manufacturing processes.
Understanding the Operational Requirements
While efficient, the use of high-intensity pulsed irradiation introduces specific operational dynamics that must be managed.
The Necessity of Precise Control
Because the synthesis occurs in under a second, there is no margin for error in the pulse duration.
The target temperature must be preset with absolute precision. An overshoot in the pulse duration could degrade the material, while an undershoot will fail to trigger the self-propagating reaction.
Material Compatibility
The primary reference highlights this process specifically for metal chalcogenides.
The success of the "self-propagating combustion" relies on the specific exothermic properties of these materials. This heating method is highly specialized for materials that can sustain this reaction once triggered.
Implications for Material Fabrication
When evaluating this technology for thermoelectric film production, consider how the heating mechanism aligns with your production goals.
- If your primary focus is throughput: This technology is ideal because it reduces synthesis time from hours or minutes to less than one second.
- If your primary focus is reaction initiation: Rely on the 100 K/s heating rate to act as a "switch" that instantly triggers the combustion reaction without thermal lag.
By leveraging tungsten halogen arrays, you move from passive heating to active, photonic reaction triggering, fundamentally changing the economics of thin-film synthesis.
Summary Table:
| Feature | Performance Specification |
|---|---|
| Energy Delivery Type | High-intensity Photonic Radiation |
| Maximum Heating Rate | ~100 K/s (Kelvin per second) |
| Synthesis Time | < 1 Second |
| Reaction Mechanism | Interlayer Self-Propagating Combustion |
| Primary Application | Metal Chalcogenide Thin Films |
Revolutionize Your Material Synthesis with KINTEK
Transition from slow, passive heating to high-speed photonic precision. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs. Whether you are synthesizing metal chalcogenides or exploring advanced thermoelectric films, our precision thermal systems provide the control and ramp rates necessary for cutting-edge research and industrial production.
Ready to accelerate your throughput? Contact us today to find your custom solution!
Visual Guide
References
- Yuxuan Zhang, Johnny C. Ho. Pulse irradiation synthesis of metal chalcogenides on flexible substrates for enhanced photothermoelectric performance. DOI: 10.1038/s41467-024-44970-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What role does the quartz tube play in a quartz heater? Unlocking Efficient Infrared Heating
- What are the physical properties of MoSi2 heating elements? Unlock High-Temperature Performance
- Why is a specifically designed radiation heater preferred over direct sample heating? | Optimize Nanomaterial Synthesis
- What are the properties of Pyrolytic Boron Nitride (PBN) when used as a heating element? Unmatched Purity for High-Temp Processes
- How does CFD simulation contribute to radiant tube design? Optimize Efficiency and Longevity with Data-Driven Science
- How can the SiO2 protective layer on MoSi2 heating elements be regenerated if it bursts off? Restore Element Performance with Expert Tips
- Why is silicon carbide used in corrosive industrial applications? Unlock Superior Durability in Harsh Environments
- What is the highest temperature heating element? Tungsten Leads, But Atmosphere is Key








